- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
In vitro Tumor Cell Migration Assay Using ThinCertsTM (Transwells)
Published: Vol 6, Iss 11, Jun 5, 2016 DOI: 10.21769/BioProtoc.1830 Views: 18836
Reviewed by: Lee-Hwa TaiAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Imaging of Human Cancer Cells in 3D Collagen Matrices
Karin Pfisterer [...] Maddy Parsons
Jan 20, 2021 6263 Views

Studying Chemotactic Migration in Dunn Chamber: An Example Applied to Adherent Cancer Cells
Khedidja Benseddik and Kossay Zaoui
Feb 5, 2022 2941 Views

Image-based Quantification of Macropinocytosis Using Dextran Uptake into Cultured Cells
Anh H. Le and Laura M. Machesky
Apr 5, 2022 4185 Views
Abstract
The high migration rate of tumor cells often results in poor prognosis for the survival of the patients. Here, we describe a protocol to measure the migration of cells using a quantitative assay. The relative tumor cell migration was measured using ThinCertsTM cell culture inserts and a lactate dehydrogenase (LDH) assay to quantify the relative cell number. The quantification of the migration with the LDH kit is much more precise than other methods using i.e. crystal blue to count the cells.
Keywords: Migration assayMaterials and Reagents
- 12 well cell culture plate (Greiner Bio One International GmbH, catalog number: 665180 )
- 96 well tissue culture test plate (TPP Techno Plastic Products AG, catalog number: 92696 )
- 1.5 ml tubes (Carl Roth GmbH + Co., catalog number: 7080.1 )
- ThinCertsTM cell culture inserts with a pore diameter of 8 µm (Greiner Bio-One GmbH, catalog number: 665638 )
- Cover slips for haemocytometer (Carl Roth GmbH + Co., catalog number: L189.1 )
- Filter tips (10 µl, 20 µl, 100 µl, 200 µl and 1,000 µl) (Carl Roth GmbH + Co., catalog number: 771288 , 774288 , 772288 , 739288 and 740288 )
- Tumor cells (the lung cancer cell lines H1975 and 2106T)
- Cell culture medium (cell line specific), with and without fetal bovine serum (FBS)
- Heat-inactivated FBS (E.U.-approved, South America Origin) (Thermo Fischer Scientific, catalog number: 10500-064 )
- 1x Dulbecco’s phosphate-buffered saline (DPBS, no calcium, no magnesium) (Thermo Fischer Scientific, GibcoTM, catalog number: 14190-094 )
- StemPro® Accutase® cell dissociation reagent (Thermo Fischer Scientific, GibcoTM, catalog number: A11105-01 )
- Mitomycin C (AppliChem GmbH, catalog number: A2190,0002 )
- Trypan blue solution (Sigma-Aldrich, catalog number: T8154 )
- 10x cell lysis buffer (Cell Signaling Technology, catalog number: 9803S )
- Cytotoxicity detection kit (LDH) (Roche Diagnostics, catalog number: 11644793001 )
Equipment
- CO2 cell incubator (Panasonic Corporation, Sanyo, model: SANYO-InCu saFe® )
- Neubauer cell counting chamber (VWR International, catalog number: BRND717810 )
- Microcentrifuge (Eppendorf AG, model: 5417R )
- Vortex mixer (VWR International, catalog number: 444-1372 )
- Versatile microplate absorbance reader (Tecan Trading AG, model: sunriseTM )
- Gilson’s Pipetman classic pipettes ( P10 , P20 , P100 , P200 , P1000 )
Procedure
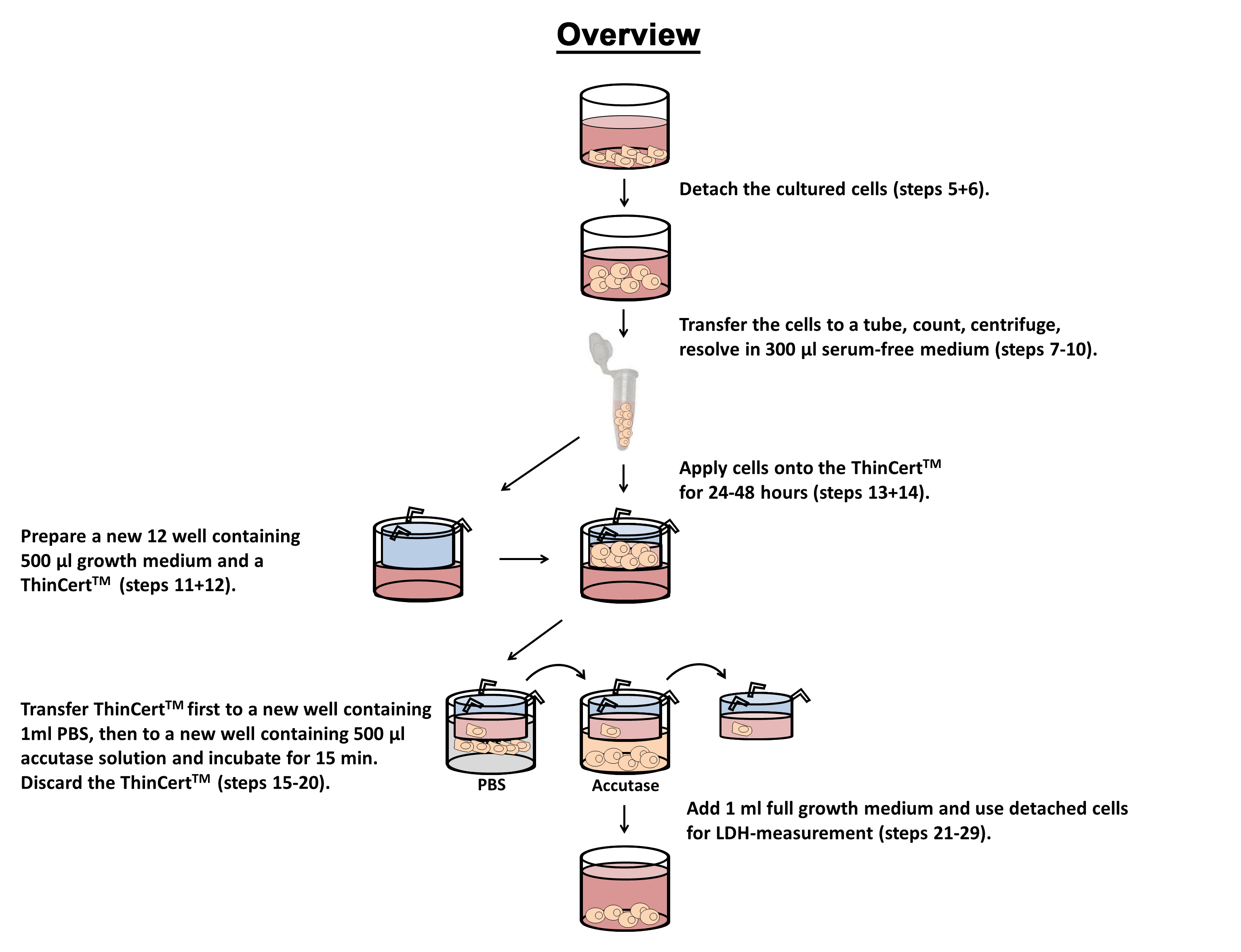
Figure 1. Flowchart of in vitro tumor cell migration assay
- Treat the cells with the desired assay (e.g., siRNA knockdown of a target gene or drug delivery) in 12 well cell culture plates.
- Replace the growth medium by 1 ml complete serum-free medium for 16 h overnight at 37 °C in the CO2 incubator.
- Treat the cells with 10 µg/ml mitomycin C in 1 ml serum-free medium for 2 h at 37 °C in the CO2 incubator to inhibit further cell proliferation.
- Wash cells once with 1 ml sterile 1x PBS.
- Add 250 µl accutase solution and incubate the cells in the incubator for approximately 5-10 min (depending on the adhesion of the used cells) to detach them.
- Add 1 ml serum-free medium to the cells, transfer the cells to a 1.5 ml tube.
- Count the cells using 10 µl cell suspension, 10 µl trypan blue and the Neubauer chamber.
- Transfer 5 x 104 cells to a new 1.5 ml tube.
- Centrifuge the cell suspension 5 min with 300 x g at room temperature.
- Remove and discard the supernatant by pipetting and add 300 µl serum-free medium, pipette up and down to resolve cell pellet.
- Take a new 12 well plate and add 500 µl of FBS containing full growth medium in each well.
- Add a ThinCertsTM to each 12 well.
- Transfer the complete cell suspension from step 10 onto a ThinCertTM.
- Let the cells migrate for 24-48 h at 37 °C in the CO2 incubator.
- Prepare a new 12 well plate with 1 ml 1x sterile PBS in each well.
- Prepare a new 12 well plate with 500 µl accutase solution in each well.
- Remove the serum-free medium in the ThinCertsTM using a pipette.
- Wash the cells first by transferring the ThinCertsTM to the plate with PBS to remove remaining medium and transfer the ThinCertsTM directly to the plate containing accutase.
- Incubate the cells in the incubator to detach them. Extend the cell specific detaching time from step 5 for additional 5 min.
- Gently tap the ThinCertsTM against the wall of the well to ensure the complete detachment of the cells; discard the ThinCertsTM.
- Add 1 ml FBS containing full growth medium to the well and transfer cells to a 1.5 ml tube.
- Centrifuge the cell suspension 5 min with 300 x g at room temperature.
- Gently remove the supernatant with a pipette and resuspend the cells with 1 ml PBS.
- Centrifuge the cell suspension 5 min with 300 x g at room temperature.
- Gently remove the supernatant, add 100 µl of 1x cell lysis buffer, vortex for 5 sec and incubate for 10 min at room temperature.
- Pellet the cells for 3 min with 13,000 x g at room temperature.
- In the meantime, prepare the LDH reaction mixture (according to the cytotoxicity detection kit manufacturer’s protocol).
- Transfer 40 µl of the cell supernatant into a 96 well plate using two technical replicates.
- Add 100 µl of the reaction mixture and incubate 10-30 min at room temperature. Protect the plate from light.
- Measure the relative LDH activity at 490 nm. The reference wavelength should be more than 600 nm.
Notes
- The migration of cell lines can vary greatly. An initial test without a treatment of the cells might be helpful, especially when different cell lines are used.
- The incubation time with the reaction mixture can be increased up to 1 h, if necessary.
- The optical density (OD490) should reach at least 0.1 to ensure a valid enzyme activity. Lower values indicate a poor migration, since some cell lines do not migrate through the ThinCertsTM (e.g., the melanoma cell line MeWo).
Acknowledgments
We would like to thank The Federal Ministry of Education and Research, Germany and the German Center for Lung Research who financially supported this study.
References
- Schneider, M. A., Granzow, M., Warth, A., Schnabel, P. A., Thomas, M., Herth, F. J., Dienemann, H., Muley, T. and Meister, M. (2015). Glycodelin: A new biomarker with immunomodulatory functions in non-small cell lung cancer. Clin Cancer Res 21(15): 3529-3540.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Schneider, M. A. (2016). In vitro Tumor Cell Migration Assay Using ThinCertsTM (Transwells). Bio-protocol 6(11): e1830. DOI: 10.21769/BioProtoc.1830.
Category
Cancer Biology > Invasion & metastasis > Cell biology assays > Cell migration
Cancer Biology > Tumor immunology > Tumor microenvironment
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link