- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Experimental Design to Determine Drought Stress Response and Early Leaf Senescence in Barley (Hordeum vulgare L.)
Published: Vol 6, Iss 5, Mar 5, 2016 DOI: 10.21769/BioProtoc.1749 Views: 17825
Reviewed by: Samik BhattacharyaAnna Дмитриевна KozhevnikovaMagdalena Migocka

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Plate Growth Assay to Quantify Embryonic Root Development of Zea mays
Jason T. Roberts [...] David M. Braun
Oct 20, 2023 2240 Views

Detection and Quantification of Programmed Cell Death in Chlamydomonas reinhardtii: The Example of S-Nitrosoglutathione
Lou Lambert and Antoine Danon
Aug 5, 2024 1577 Views

Enzymatic Starch Quantification in Developing Flower Primordia of Sweet Cherry
Nestor Santolaria [...] Afif Hedhly
Apr 5, 2025 1885 Views
Abstract
Premature leaf senescence induced by drought stress is a main factor for yield losses in barley. Research in drought stress tolerance has become more important as due to climate change the number of drought periods will increase and tolerance to drought stress has become a goal of high interest in barley breeding. However, reliable screening for drought stress tolerance is still a difficult task. This protocol describes the experimental design for the phenotyping for drought stress tolerance and early leaf senescence in the juvenile stage of barley (A) and the determination of six physiological parameters involved in drought tolerance and leaf senescence (B to G) according to Wehner et al., (2015).
Keywords: Barley
A. Experimental design
Materials and Reagents
- Sticks for labels (Hermann Meyer KG, catalog number: 180230 )
- Plastic sticks (Hermann Meyer KG, catalog number: 180206 )
- Rubber binder (Hermann Meyer KG, catalog number: 321234 )
- Barley seeds
- 70% white peat
- 30% clay
- N (nitrogen power)
- P2O5
- K2O
- Mixed clay soil ED73 (H. Nitsch & Sohn GmbH & Co. KG) (see Recipes)
Equipment
- Greenhouse facility
- Movable greenhouse benches (80 x 100 cm)
- Square pots (16 x 16 x 16 cm) (Hermann Meyer KG, catalog number: 720016 )
- Beaker for watering (VWR International, catalog number: 213-3402 )
- Labels (Baumann Industries, catalog number: 2.508.003 )
- Weighing scale (KERN & SOHN GmbH)
- Compartment dryer (Heratherm oven) (Thermo Fisher Scientific)
Procedure
- Adjust greenhouse by heating, ventilation and lighting for long day conditions in a temperature range from 20 to 22 °C at day (16 h) and 17 to 19 °C at night (8 h). If natural radiation is below 20 klx, additional light should be supplied from 6 am to 10 pm and above 30 klx shading should be used (light intensity of additional illumination at pot height is ~10 klx).
- Homogenize content of different bags of ED 73 soil to optimize comparability between the pots.
- In correspondence to respective humidity fill square pots with a defined weight of the soil (in our case 1,500 x g at 40% dry substance of the soil).
- Label pots and sow ten seeds of each barley genotype per pot (in our case three replications for control and drought stress treatment for 156 genotypes). For better visibility labels could be pinned on label stakes (Figure 1).
- Put 20 pots ordered by the number of genotypes at each greenhouse bench according to the experimental design chosen (in our case a split plot design). Control and stress treatment are allocated to separate benches (Figure 1).
- Water all pots up to 70% of the maximal soil water capacity (WC). The weight of added water is calculated out of the saturated soil weight and dry weight according to DIN ISO 11465 1996-12 (Paech and Simonis, 1952) at three exemplary pots as follows:
- Put gauze at the bottom, fill three pots with soil and add water till saturation.
- Wait for 6 h till water is drained by gravity and weight.
- Dry the soil (two days in compartment dryer) at 105 °C and weigh.
- 100% WC is calculated out of the weight of saturation minus the dry weight.
- The weight to which the pot is watered corresponds to 70% (control) or 20% (drought stress) WC plus the dry weight and the pot weight.
- Put gauze at the bottom, fill three pots with soil and add water till saturation.
- Water every day to 70% WC by weighing.
- After germination, reduce seedlings to seven plants per pot.
- To minimize effects of plant position, movable benches should be moved every day.
- Drought stress starts at the primary leaf stage [BBCH 10, according to Stauss (1994)] seven days after sowing (das). At this time primary leaves of the juvenile barley plants are fully expanded. Stop watering of the stress variant till the pot weight reaches 20% WC. Check this by weighing ten exemplary stressed pots over the time of the experiment.
- Then keep this level by weighing each pot and re-water to the weight of 20% WC daily.
- Water control plants daily to 70% WC (weight of the plants is neglected).

Figure 1. Pot experiment in greenhouse (10 days after sowing) - At 36 das (BBCH 33) a four weeks stress period is reached and physiological parameters are determined as well as biomass is harvested according to protocol B to G below. For analysing physiological traits primary leaves should be used because at these oldest leaves drought stress induced leaf senescence occurs first (Figure 2).

Figure 2. Primary leaves differentiating in drought stress response regarding leaf senescence
Notes
In our pot experiments it turned out, that it is necessary to measure parameters for leaf senescence in a separate pot experiment, because only in this way it was possible to separate the effects of drought stress induced leaf senescence and age-related leaf senescence from light deficiency induced leaf senescence. To achieve this, the shadowing effects of other leaves on the primary leaf are minimized by tying up all barley leaves (with rubber binder on stakes) about 14 das, except the primary leaf (Figure 3A). General settings are the same as described above, but only four plants are grown in smaller pots (12 x 12 x 12 cm with 550 g soil) and control and stress treated pots are mixed on the greenhouse benches in rows (Figure 3B).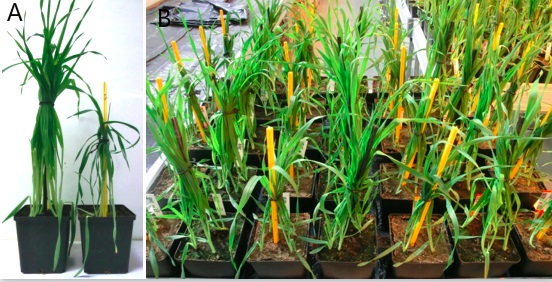
Figure 3. Barley plants with tied up leaves. A. Single plants at 36 days after sowing under control (left) and drought stress treatment (right). B. One greenhouse bench at 20 days after sowing with four rows stress treatment (yellow sticks) and three rows control treatment (black sticks).
Recipes
- Mixed clay soil ED73
70% white peat
30% clay with pH ~6 and 1 kg/m³
Mineral nutrients- 14% N
- 16% P2O5
- 18% K2O
- 20% N
- 10% P2O5
- 15% K2O
- 14% N
B. Leaf senescence: Leaf colour
Materials and Reagents
- Barley plants grown as described above
Equipment
- Chlorophyll Meter SPAD-502 Plus (Konica Minolta)
Procedure
- Measure leaf colour directly in the greenhouse on the primary leaves at 33-34 days after sowing (das).
- With Minolta SPAD readings leaf colour representing the status of leaf senescence is measured non destructive.
- After calibration by an empty clip, take five evenly distributed readings on each of three primary leaves (three barley plants) per pot which are averaged by the SPAD-Meter.
- At each primary leaf, measurement should be done at the upper side of the leaf and in the middle of the leaf avoiding to clip the middle leaf-vein (Figure 4).

Figure 4. Positions for measurement of leaf colour on three primary leaves
C. Leaf senescence and drought tolerance: Electron transport rate at PSII
Materials and Reagents
- Barley plants grown as described above
Equipment
- OS1p-Chlorophyll fluorometer (Opti-Sciences)
Procedure
Determine the electron transport rate (ETR) at photosystem II (PSII) as a parameter for leaf senescence at 34-35 das. With the portable OS1p (Figure 5) chlorophyll fluorescence is measured non destructive on light adapted plants. Because this analysis is very light sensitive, measurement should be conducted exactly at the position where the pot is located on the greenhouse bench and the shading of the greenhouse should be used to ensure continuous lighting conditions during all measurements. Besides, measurement should be done not before 10 am and not after 3 pm (around noon) receiving the optimum of daily photosynthesis. 
Figure 5. OS1p-Chlorophyll fluorometer. The display shows three exemplary measurements down right and general settings top left. Besides this, the leaf clip for measurement is shown consisting out of the clip with the trigger button and the sensor for detecting the photosynthetic active radiation (PAR).
For measurement with the fluorometer first select the photosynthetic yield menu Y(II).
Adjust modulation intensity at 50%, saturation intensity at 100% and for the ETR calculation include the formula ETR = Y(II) * PAR * 0,84 * 0.5. Input values for constants representing light which is absorbed by the leaf (0.84) and light which is equally absorbed by PSI and PSII (0.5) in addition to the measured photosynthetic active radiation (PAR) and the quantum photosynthetic yield of PSII [Y(II)] (Krall and Edwards, 1992).
Take three measurements for each pot in the middle of three primary leaves at the upper side of the leaves. Put the leaves in the clip with the leaf margin at the bottom of the clip. Then wait till the light impulses are finished indicated by a peep.
Data dumps are stored in the memory of the fluorometer and can be read out later by USB transfer and averaged for each pot.
Notes
The Fluorometer also gives values for Y(II), but here the ETR (µmol/ m²s) is used because the PAR is included to compensate potential lighting variations.
D. Drought tolerance: Total content of soluble sugars
Materials and Reagents
- Pipette (1,000 µl/10 ml) (Eppendorf/Finnpipette Labsystems)
- Tubes (15 ml) (VWR International, catalog number: 525-0308 )
- Tubes (2 ml) (VWR International, catalog number: 211-2120 )
- Test tubes (glass) (VWR International, catalog number: 4.902.002.75 )
- Barley plants grown as described above
- Liquid nitrogen
- D-Glucose (VWR International, catalog number: 1.08337.0250 )
- Polyvinylpolypyrrolidone (PVPP) (VWR International, catalog number: 1.07302.0100 )
- Distilled water
- Sulfuric acid 100% (VWR International, catalog number: 1.00713.1000 )
- Borosilicate glass bottle (brown) (VWR International, catalog number: 215-2328 )
- Anthrone (VWR International, catalog number: 1.01468.0010 )
- Thiourea (VWR International, catalog number: 1.07979.0250 )
- Sulfuric acid 70% (see Recipes)
- Anthrone reagent (see Recipes)
Equipment
- Weighing scale (KERN & SOHN GmbH)
- Scissors
- Styropor box
- Freeze dryer (Christ Alpha 1-4 LD plus)
- Water bath (GFL)
- Shaker (Vortex Genie 2) (Scientific industries)
- Test tube racks (Geyer GmbH & Co, catalog number: 9.905 970 )
- Glass marbles (toy store, diameter of 15 mm)
- Centrifuge (23,000 x g) (Tuttlingen Germany, model: Hettich Universal 16R )
Note: Currently, it is “Gemini BV Laboratory, model: Hettich Universal 16R”. - Cuvette (VWR International, catalog number: 634-9014 )
- Spectrophotometer (Thermo Fisher Scientific, model: Genesys 10S UV VIS )
- Beaker (VWR International, catalog number: 213-1131 )
Procedure
First, prepare a standard curve for glucose content which should be repeated for every anthrone reagent.
- Prepare five different dilutions of D-Glucose (each 500 µl) in five test tubes: 0 µM, 50 µM, 100 µM, 150 µM and 200 µM.
- Slowly add for each test tube 2.5 ml anthrone reagent by vortexing till a clear solution is present.
- Continue with steps 12-14 together with the samples.
- After measuring draw the standard curve for the glucose content (Figure 6).
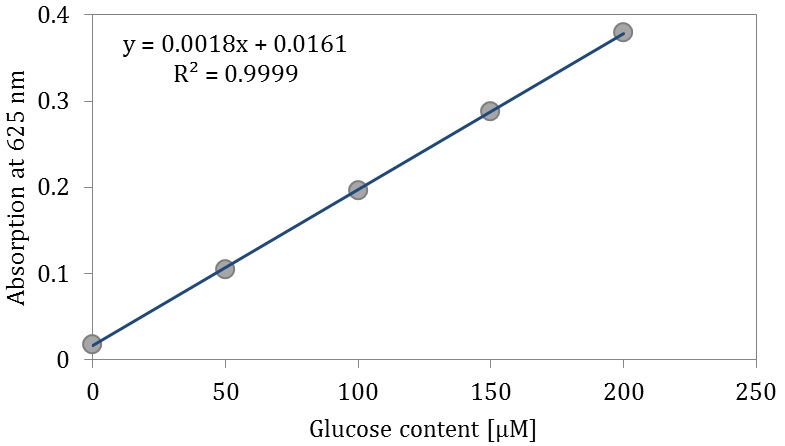
Figure 6. Standard curve for glucose content
At 36 das samples for analysis of the total content of soluble sugars are taken and analysed according to Yemm and Willis (1954). - Cut 1 cm pieces from the middle of five primary leaves per pot (100 -200 mg).
- Weigh these (fresh weight is only used to calculate dry weight content for osmolality), fill in tubes (15 ml) as pooled samples per pot and freeze in liquid nitrogen using a styropor box.
- Samples are then freeze dried and reweighed (dry weight).
- Add 4 ml distilled water (depends on the amount of leaf tissue sampled) and boil the samples in the water bath for 30 min by 100 °C (use the test tube racks for water baths).
- After cooling down, add 1,000 µl of the sample solution to tubes (2 ml) filled with 0.05 g PVPP and vortex.
- Centrifuge the tubes for 5 min at 23,000 x g (5,000 rpm).
- Transfer 200 µl of the supernatant solution to test tubes and add 300 µl distilled water.
- By vortexing slowly add 2.5 ml of the anthrone reagent till a clear solution is present.
- Put on each glass tube a glass marble (Figure 7) and boil the solutions in the water bath for 15 min at 100 °C (use the test tube racks for water baths).
- Cool down in a cold water bath (20 °C) for 10 min.
- Samples (Figure 7) are measured directly (maximum in 1 h time) by a spectrophotometer at 625 nm using a cuvette. Zero calibration should be done with distilled water.

Figure 7. Test tubes ready for measurement of total content of soluble sugars. Exemplarily, three test tubes with samples for control (light blue, left) and three test tubes with samples for drought stress (dark blue, right) treatment are shown. - Total content of soluble sugars is calculated using the standard curve and set in relation to dry weight (µmol/g).
Notes
The sample volume of 200 µl taken in step D11 is varying for different developmental stages of barley and also for different crops and depends on the amount of leaf tissue sampled. Important is, that the final volume in step D11 is 500 µl. Volume should be defined for the optimum range of the standard curve.
Recipes
- 70% sulfuric acid (mix in a brown bottle and incubate for 12 h in dark)
70 ml sulphuric acid (100%)
30 ml distilled water - Anthrone reagent (stable for one day)
Sulphuric acid (70%)
920 mg/l anthrone
920 mg/l thiourea
E. Drought tolerance: Content of free proline
Materials and Reagents
- Aluminium foil
- Tubes (15 ml) VWR International, catalog number: 525-0308 )
- Stopper (VWR International, catalog number: 217-0511 )
- Pipette (1,000 µl/10 ml) (Eppendorf/Finnpipette Labsystems)
- Barley plants grown as described above
- Liquid nitrogen
- L-Proline (VWR International, catalog number: 1.07434.0010 )
- Toluol (VWR International, catalog number: 1.08325.1000 )
- Distilled water
- Ninhydrin (VWR International, catalog number: 1.06762.0100 )
- Glacial acetic acid (AppliChem, catalog number: A0820.2500 )
- Ninhydrin reagent (see Recipes)
Equipment
- Weighing scale (KERN & SOHN GmbH)
- Scissors
- Styropor box
- Freeze dryer (Christ Alpha 1-4 LD plus)
- Water bath (GFL)
- Test tubes (glass) (VWR International, catalog number: 4.902.002.75 )
- Test tube racks (Geyer GmbH & Co, catalog number: 9.905 970 )
- Glass marbles (toy store, diameter of 15 mm)
- Shaker (Scientific industries, model: Vortex Genie 2 )
- Magnetic mixer (RCT basic) (IKA Labortechnik)
- Fortuna® Optifix® Safety Dispenser (Sigma-Aldrich, catalog number: Z260207 )
- Fume hood
- Cuvette (VWR International, catalog number: 634-9014 )
- Spectrophotometer (Thermo Fisher Scientific, model: Genesys 10S UV VIS )
- Beaker (VWR International, catalog number: 213-1131 )
Procedure
First, prepare a standard curve for proline content which should be repeated for every ninhydrin reagent.
- Prepare five different dilutions of L-Proline (each 500 µl) in five test tubes: 0 µM, 25 µM, 50 µM, 75 µM and 100 µM.
- Slowly add for each test tube 2 ml ninhydrin reagent by vortexing till a clear solution is present.
- Continue with steps D11-16 together with the samples.
- After measuring draw the standard curve for the proline content (Figure 8).
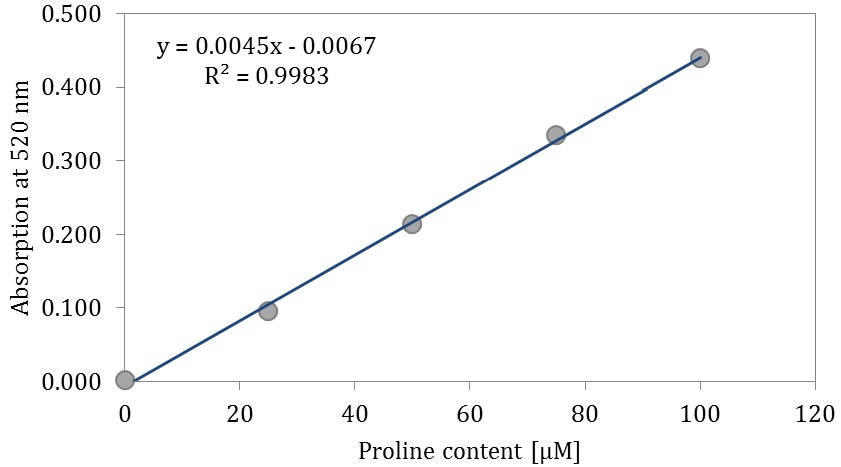
Figure 8. Standard curve for proline content
At 36 das samples for analysis of the content of free proline are taken and analysed according to Bates et al. (1973). For this analysis, the same sample solution can be used as for the total content of soluble sugars because steps 5-8 are the same. - Cut 1 cm pieces from the middle of five primary leaves per pot (100 -200 mg).
- Weigh these (fresh weight is only used to calculate dry weight content for osmolality), fill in tubes (15 ml) as pooled samples per pot and freeze in liquid nitrogen using a styropor box.
- Samples are then freeze dried and reweighed (dry weight).
- Add 4 ml distilled water (depends on the amount of leaf tissue sampled) and boil the samples in the water bath for 30 min at 100 °C (use test tube racks for water baths).
- After cooling down, 1,000 µl of the sample solution are filled in a test tube, each.
- By vortexing slowly add 2 ml of the ninhydrin reagent till a clear solution is present.
- Put on each test tube a glass marble (see Figure 7) and boil the solutions in the water bath for 15 min at 100 °C (use test tube racks for water baths).
- Cool down in a cold water bath (20 °C) for 10 min.
Use a laboratory fume hood for the next steps: - Remove the glass marble and add 5 ml toluol (e.g. with a dispenser).
- Close the test tube with a stopper and vortex for 15 sec.
- Incubate the samples for the next 90 min in darkness (up to 4 h are possible) at room temperature.
- Two phases occur (Figure 9) from which the upper one is now transferred to a cuvette for measurement with a spectrophotometer at 520 nm. Zero calibration should be done with toluol.

Figure 9. Test tubes ready for measurement of content of free proline. Exemplarily, three test tubes with samples for control (light red, left) and three test tubes with samples for drought stress (dark red, right) treatment are shown. - Content of free proline is calculated using the standard curve and set in relation to the dry weight (µmol/g).
Notes
In step E9, 1,000 µl of the sample solution is used. This volume is varying for different developmental stages of barley and also for different crops and depends a lot on the amount of leaf tissue sampled. Volume should be defined for the optimum range of the standard curve.
Recipes
- Ninhydrin reagent (stable for one day, mix in dark, cover a beaker with aluminium foil and dissolve on a magnetic mixer)
0.5 g ninhydrin
20 ml distilled water
30 ml glacial acetic acid
F. Drought tolerance: Osmolality
Materials and Reagents
- Tubes (2 ml) (VWR International, catalog number: 211-2120 )
- Pipette (100 µl/200 µl) (Eppendorf)
- Barley plants grown as described above
- Liquid nitrogen
- Distilled water
- Calibration standard for the osmomat (Gonotec GmbH, catalog number: 30.9.0020 )
Equipment
- Weighing scale (KERN & SOHN GmbH)
- Scissors
- Styropor box
- Tweezer
- Swing mill (Retsch, model: MM200 )
- Grinding balls (VWR International, catalog number: 412-0070 )
- Grinding jars (Retsch, catalog number: 22.008.0005 )
- Shaker (Scientific industries, model: Vortex Genie 2 )
- Centrifuge (21,000 x g) (Wehingen, model: Hermle Z233MK2 )
Note: Currently, it is “HERMLE Labortechnik GmbH, model: Hermle Z233MK2”. - Osmomat (Gonotec GmbH, model: Osmomat 3000 Gonotec )
- Tubes for the osmomat (Gonotec GmbH, catalog number: 30.9.0010 )
Procedure
At 36 das the osmolality is determined by comparative measurements of the freezing points of distilled water and the cell sap.
- Cut 1 cm pieces from the middle of five primary leaves per pot.
- Weigh these, fill pooled samples per pot in tubes (2 ml) and freeze in liquid nitrogen using a styropor box.
- Put also the grinding balls and grinding jars into the liquid nitrogen (into the styropor box).
- Take each tube and fill in two grinding balls with a tweezer.
- Take off the grinding jars and fill them with five tubes each.
- Grind the leaves in the swing mill for 3 min (30/sec).
- Add 200 µl distilled water into the tubes and vortex them.
- Take the grinding balls out of the tubes with the tweezer.
- Centrifuge the tubes for 15 min at 21,000 x g (15,000 rpm) by 4 °C.
- Pipette the supernatant cell sap in new tubes (2 ml).
For measurement with the osmomat use the original tubes for the osmomat. - Calibrate the osmomat with 15 µl calibration standard till a value of exactly 0.3 is reached.
- Adjust a zero value with 15 µl distilled water.
- Measure 15 µl cell sap each.
- Values (osmol/kg) can be corrected for the water content (difference of fresh and dry weight). Dry weight is calculated in relation to the dry weight content of the samples for the determination of content of free proline and the total content of soluble sugars (see above).
G. Drought tolerance: Biomass yield
Materials and Reagents
- Barley plants grown as described above
Equipment
- Weighing scale (KERN & SOHN GmbH)
- Crispack bags (Baumann, catalog number: 3.331.100 )
- Scissors
- Compartment dryer (Heratherm oven) (Thermo Fisher Scientific)
Procedure
- At 36 das cut the whole biomass above ground per pot.
- Put biomass for each pot with the label in one crispack bag.
- Close bags.
- Dry the biomass within the bags in a compartment dryer at 105 °C until weight doesn’t change (about two days with ventilation).
- By weighing the dry weight, total biomass yield (g) is detected.
Acknowledgments
The authors thank the Interdisciplinary Center for Crop Plant Research (IZN) of the Martin-Luther-University of Halle-Wittenberg for funding the project. Furthermore, some of the protocols are modified or adapted from previous work acknowledging Bates et al. (1973), Paech and Simonis (1952) as well as Yemm and Willis (1954).
References
- Bates, L. S., Waldren, R. P. and Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant Soil 39:205-207.
- Krall, J. P. and Edwards, G. E. (1992). Relationship between photosystem II activity and CO2 fixation in leaves. Pysiol Plantarum 86:180-187.
- Paech, K. and Simonis, W. (1952). Pflanzenphysiologische Praktika Band I Übungen zur Stoffwechselphysiologie der Pflanzen. Springer Verlag, Berlin Göttingen Heidelberg.
- Stauss, R. (1994). Compendium of growth stage identification keys for mono-and dicotyledonous plants: Extended BBCH scale. Ciba.
- Wehner, G. G., Balko, C. C., Enders, M. M., Humbeck, K. K. and Ordon, F. F. (2015). Identification of genomic regions involved in tolerance to drought stress and drought stress induced leaf senescence in juvenile barley. BMC Plant Biol 15: 125.
- Yemm, E. W. and Willis, A. J. (1954). The estimation of carbohydrates in plant extracts by anthrone. Biochem J 57(3): 508-514.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Wehner, G., Balko, C. and Ordon, F. (2016). Experimental Design to Determine Drought Stress Response and Early Leaf Senescence in Barley (Hordeum vulgare L.). Bio-protocol 6(5): e1749. DOI: 10.21769/BioProtoc.1749.
Category
Plant Science > Plant physiology > Abiotic stress
Plant Science > Plant physiology > Photosynthesis
Plant Science > Plant physiology > Plant growth
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











