- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Craniotomy for Cortical Voltage-sensitive Dye Imaging in Mice
Published: Vol 6, Iss 3, Feb 5, 2016 DOI: 10.21769/BioProtoc.1722 Views: 14680
Reviewed by: Soyun KimManuel SarmientoTifany Desprez

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Fluorescent Measurement of Synaptic Activity Using SynaptopHluorin in Isolated Hippocampal Neurons
Hongmei Li [...] Elizabeth A. Jonas
Dec 5, 2014 12158 Views

Simultaneous Microendoscopic Calcium Imaging and EEG Recording of Mouse Brain during Sleep
Sasa Teng and Yueqing Peng
May 5, 2023 2487 Views

Construction of Large Cranial Windows With Nanosheet and Light-Curable Resin for Long-term Two-Photon Imaging in Mice
Taiga Takahashi [...] Tomomi Nemoto
Jul 5, 2025 2076 Views
Abstract
Cortico-cortical interactions play crucial roles in various brain functions. Here, we present a detailed surgical procedure for cortical voltage-sensitive dye (VSD) imaging that allows monitoring of spatiotemporal dynamics in cortical activity in living mice. Cortical neurons in the upper layers (layer 1-3) are stained with a VSD, and an image sensor with a fast sampling rate (500 Hz) detects fluorescent changes in corrective activity. The procedure includes fixing a mouse brain to a stereotaxic apparatus, craniotomy on a large cortical area, VSD staining, and wide-field imaging of cortical activity. The entire procedure can be completed in 5 h (from the administration of anesthesia to the start of cortical VSD imaging).
Keywords: Voltage-sensitive dye imagingMaterials and Reagents
- Fine needle (Bonn Micro Probes) (Fine Science Tools, catalog number: 10030-13 )
- Cover glass (Matsunami Glass Ind, catalog number: C024241 )
- Wild-type mice (Japan SLC, model: C57BL / 6JJmsSlc )
- Isoflurane (e.g., Wako Pure Chemical Industries, catalog number: 099-06571 )
- Lidocaine solution (80 mg/ml) (e.g., Xylocaine Pump Spray 8%) (AstraZeneca) for local anesthesia
- Dental cements (Super-Bond C&B) (Sun medical) (GC's Global, UNIFAST II)
- Voltage-sensitive dye (OPTICAL IMAGING LTD, catalog number: RH1691 )
- NaCl
- KCl
- MgCl2.6H2O
- CaCl2.2H2O
- HEPES
- Distilled water
- RH1691
- Ringer’s solution (see Recipes)
- Voltage-sensitive dye (VSD) solution (see Recipes)
Equipment
- Anesthesia system for isoflurane (Shinano, catalog number: SN-487-0T )
- Electric clipper (e.g., Panasonic Corporation, catalog number: ER803P ) for cutting mouse hair
- Feedback-controlled heating pad (Bio Research Center, catalog number: BWT-100A )
Note: The pad monitors the mouse body temperature and maintains it at the desired temperature. - Head holder (stereotaxic apparatus) (NARISHIGE Group, model: SG-4N )
- Fine scissors (Fine Science Tools, catalog number: 91460-11 )
- Student Dumont #7 Forceps (Fine Science Tools, catalog number: 91197-00 )
- Dumont #5SF Forceps (Fine Science Tools, catalog number: 11252-00 )
- Cotton swab for absorbing the ringer’s solution
- Vacuum pump (e.g., AGC TECHNO GLASS CO., model: VPUMP-140 ) for removing the ringer's solution
- Surgical blade (e.g., Kai industries, catalog number: 310-A )
- Head-fixation plate (handmade, Figure 1A)
- Plate holder (custom-made, Figure 1B)
- Stereo Microscope (OLYMPUS, model: SZX7 )
- Green LED light (REVOX Inc., model: SLG-50S-G )
- Dental drill (SHOFU Inc., model: Tas-35LX )
- Dental round bur (SHOFU Inc., model: ELA Steel Bur HP-1 )
- Lens blower (e.g., HAKUBA, model: KMC-45 )
- Ultra-Fast CMOS Imaging System (Brainvision, model: MiCAM ULtima )
Note: For reference imaging (see procedure step 13), the cortex is illuminated with a blue LED light (center wavelength: 465 nm) through a 506-nm dichroic mirror; green fluorescence is corrected through a 536/40-nm filter.
For voltage-sensitive dye imaging, VSD fluorescence is excited with a red LED light (center wavelength: 625 nm). The excitation light is filtered with a 632/22-nm band-pass filter, reflected using a 655-nm dichroic mirror, and focused 375 μm below the cortical surface. Fluorescence is filtered with a 665-nm long pass filter. - Blue LED light (Brainvision, model: LEX2-B )
- Red LED light (REVOX Inc., model: SLG-50S-R )
- Stimulator (e.g., NIHON KOHDEN CORPORATION, model: SEN-5201 )
- Metal electrodes for hindpaw stimulation (handmade, Figure 1C)
- Vortex mixer (e.g., Scientific Industries Inc., model: VORTEX-GENIE 2 )
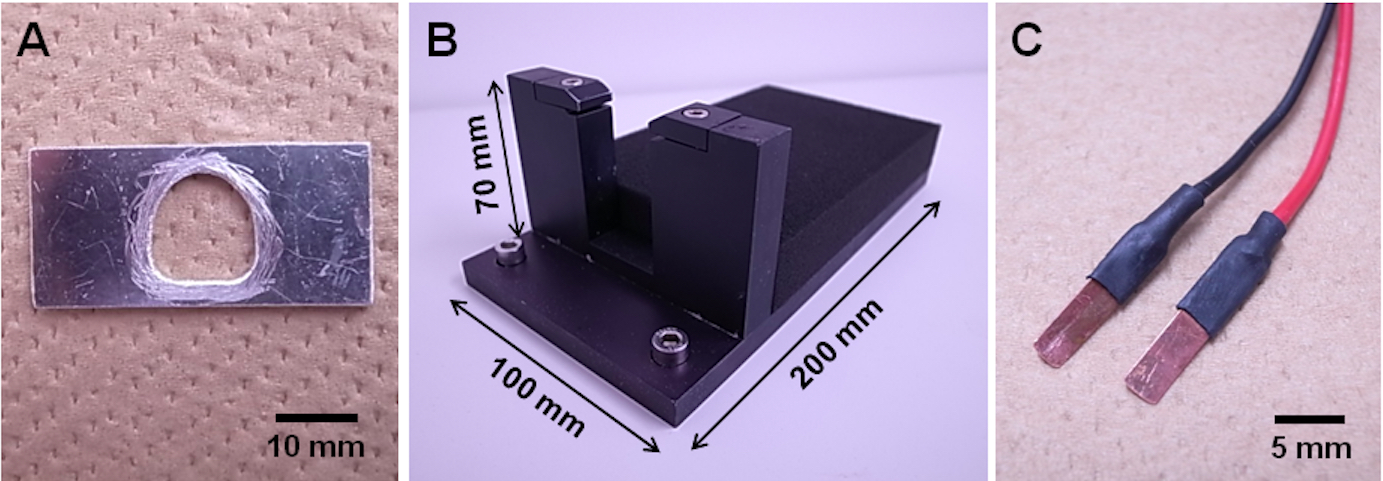
Figure 1. Head-fixation equipment. A. Handmade head-fixation plate; B. Custom-made plate holder; C. Handmade metal electrodes for hindpaw stimulation.
Software
- Acquisition software (Brainvision, model: UL-Acq)
- Analysis software (Brainvision, model: BV_Ana)
Procedure
All animal experiments were performed in accordance with institutional guidelines and were approved by the Animal Experiment Committee from the RIKEN BSI.
- Preparation for surgery (timing, 60-70 min)
- Anesthetize the mouse with isoflurane (1-2%, vol/vol in air).
- Cut the hair on the mouse’s head with an electric clipper.
- Place the mouse onto a heating pad (37 °C), temporarily stabilize the head with ear bars of a head holder, and insert the nostril into a tube for anesthetizing (Figure 2A). We did not use the nose clamp of the head holder.
- Using scissors, remove the skin (about 1 cm in diameter) covering the skull of both hemispheres (Figure 2B).
- Apply lidocaine solution to the wound.
Note: Do not apply lidocaine to the brain because it blocks the voltage-gated sodium channels and will suppress the VSD response. - Gently remove the periosteum with a surgical blade by scraping the skull (Figure 2B). This will help the glue adhere to the bone.
- Glue a head-fixation plate onto the exposed skull with dental cement (Super-Bond C&B) (Figure 2C). Do not allow any gaps between the skull and cement to prevent solution leakage.
- After drying out the dental cement (about 10 min), transfer the mouse to a plate holder, fix the head-fixation plate in the plate holder, and maintain body temperature at 37 °C with the heating pad (Figure 2D).
- Make a chamber by heavily coating and piling up dental cement (UNIFAST II) as a bank (about 1 or 2 mm in height) around the head-fixation plate hole (Figure 2E). This chamber will be used for staining by filling the chamber with voltage-sensitive dye (VSD) solution (step D15).
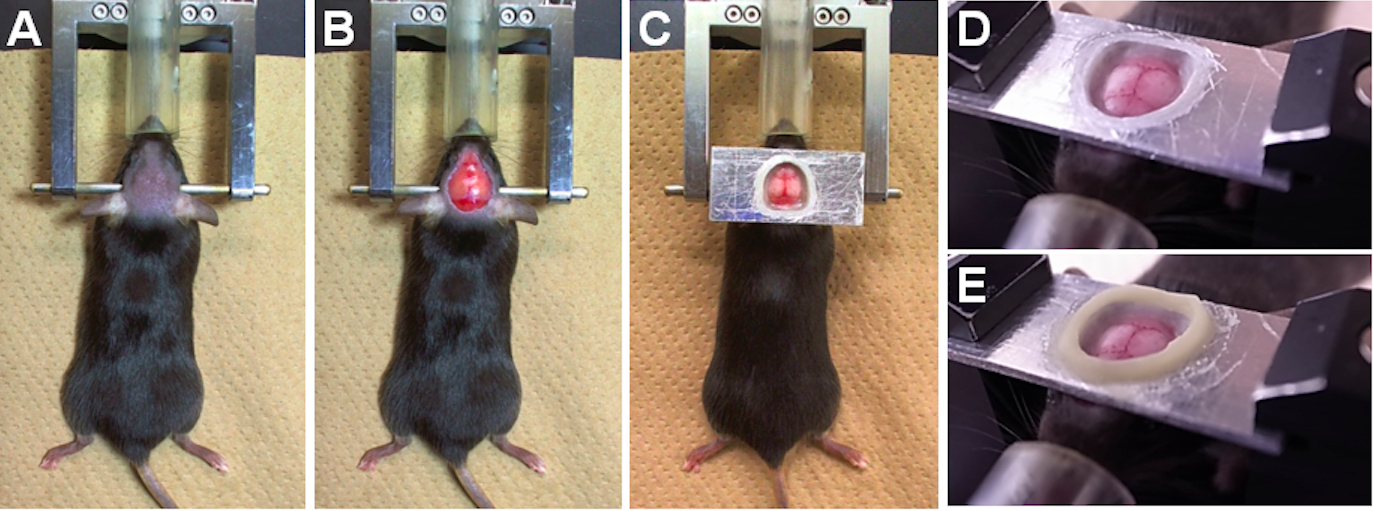
Figure 2. Experimental preparation for the craniotomy. A. The hair on the head has been cut. B. The skin and periosteum have been removed. C. The head-fixation plate has been attached onto the exposed skull. D. The mouse has been transferred to a plate holder, and the head-fixation plate has been fixed. E. The chamber has been created with dental cement onto the head-fixation plate.
- Anesthetize the mouse with isoflurane (1-2%, vol/vol in air).
- Craniotomy (timing, 20-40 min)
- Perform a craniotomy over a large portion of the right hemisphere (details are shown below). During the craniotomy, a green LED light source (center wavelength: 525 nm) can help visualize blood vessels to avoid surgical damage. We did not control brain swelling.
- Using a dental drill (head diameter: 0.8 mm), make a groove by scraping the skull and an island in the right hemisphere (Figure 3A).
Note: To prevent the heat damage generated by drilling, you should not drill the skull at the same spot continuously (5 sec maximum). It is helpful to cool down the drilled area with Ringer’s solution or by using an air blower.
Note: The groove does not penetrate the skull at this step. - Check the grooved skull thickness by gently pushing on the island with the fine forceps.
- If the island moves up and down when lightly pushing, carefully scratch the groove with a fine needle in order to cut the grooved skull.
- Filling the chamber with a ringer’s solution for wetting the skull, which allows us to easily remove the island from the skull.
- Remove the island from the rest of the skull using the fine forceps (Figure 3B and 3D).
Note: Do not touch the brain, and leave the dura matter intact to avoid damage. When the cortical blood vessels are damaged, you can see the blood spread under the dura matter. In this case, you should prepare a new mouse and repeat the procedure. - Gently wash the exposed cortex with the ringer’s solution until the superficial cortical vessels become clearly visible (Figure 3C).
- If the blood on the dura mater cannot be removed with the wash, gently dry the exposed dura with a lens blower and repeat steps B10f-g until clear (Figure 3C).
Note: This procedure is helpful for stopping the bleeding.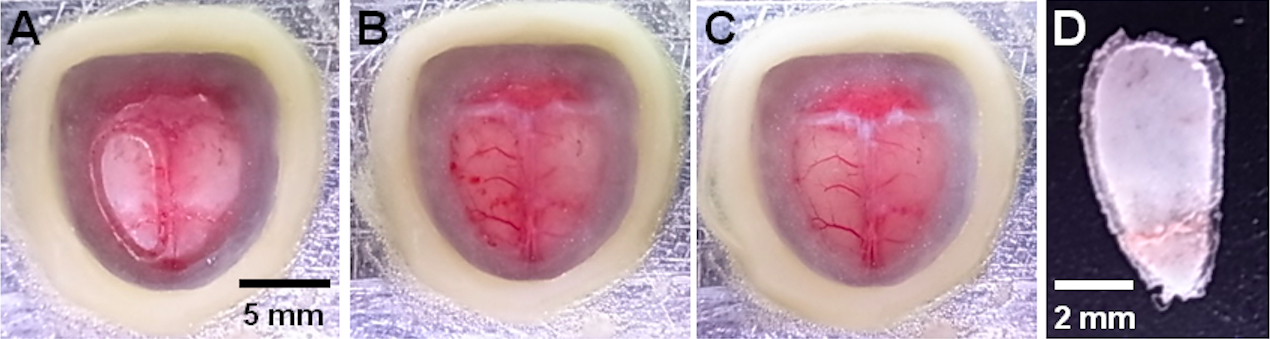
Figure 3. Craniotomy for wide-field cortical VSD imaging. A. A grooved skull (right hemisphere). B. The superficial blood vessels immediately after the craniotomy. C. The blood vessels after washing. D. The removed skull.
- Using a dental drill (head diameter: 0.8 mm), make a groove by scraping the skull and an island in the right hemisphere (Figure 3A).
- Transfer the mouse to a recording setup (an epi-fluorescent microscope) with the heating pad, fix the head-fixation plate, and reduce isoflurane concentration to 1.0% (vol/vol in air) for anesthetized experiments. For experiments with awake animals, the following steps are performed without anesthesia.
Note: Heart rate should be monitored and the anesthesia level should be controlled to prevent unforeseen death and to get a good response.
- Perform a craniotomy over a large portion of the right hemisphere (details are shown below). During the craniotomy, a green LED light source (center wavelength: 525 nm) can help visualize blood vessels to avoid surgical damage. We did not control brain swelling.
- Finding a region of interest (ROI) for imaging (timing, 10 min)
- Fill the chamber with the ringer’s solution, and place a thin cover glass over the solution.
- Search an ROI with the fluorescent microscope and capture the ROI image before VSD imaging. This is because VSD staining during the next step darkens the cortex (Figure 4), and researchers are unable to precisely create an activity map on the cortex.
Note: Positions of superficial blood vessels on the cortex can be a reference for the response area (Figure 5).
- Fill the chamber with the ringer’s solution, and place a thin cover glass over the solution.
- VSD staining (timing, 2.5 h-3 h)
- Remove the cover glass, and suck the ringer’s solution out of the chamber.
- Fill the chamber with VSD solution, and place a thin cover glass over the chamber to avoid drying (Figure 4A).
- Overlay an aluminum sheath onto the chamber to keep the chamber out of the light (Figure 4B).
Note: Be careful not to allow air bubbles to enter the VSD solution to prevent irregular staining. - Apply the VSD solution for 90 min so that the dye solution reaches the cortex through the dura matter.
- Remove the VSD solution, and rinse the chamber every 15 min with a fresh ringer’s solution to remove unbound dye until the solution becomes clear (60-90 min).
- After washing, fill the chamber with the ringer’s solution, and place a thin cover glass over the solution (Figure 4C).
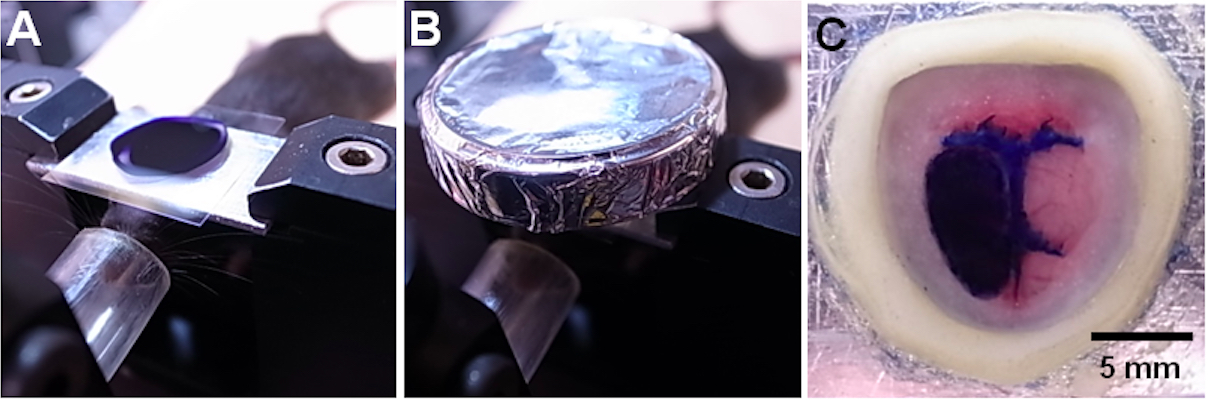
Figure 4. VSD staining. A. The chamber filled with VSD solution was covered with a thin cover glass. B. The chamber was covered by an aluminum sheath. C. After washing the cortex, the craniotomy is filled with the ringer’s solution and covered with a thin cover glass.
- Remove the cover glass, and suck the ringer’s solution out of the chamber.
- Cortical VSD imaging (timing, depending on your experiment)
- Record cortical activity (Figure 5A). Electrically stimulate the contralateral hindpaw 3 sec after starting the imaging, and monitor the VSD responses for 4 sec (3 sec before stimulation and 1 sec after stimulation) at every imaging (Figure 5B). By referencing the positions of blood vessels captured beforehand (step C13; Figure 5C), overlay the cortical activity map and vessel positions in order to identify a functional cortical map (Figure 5D).
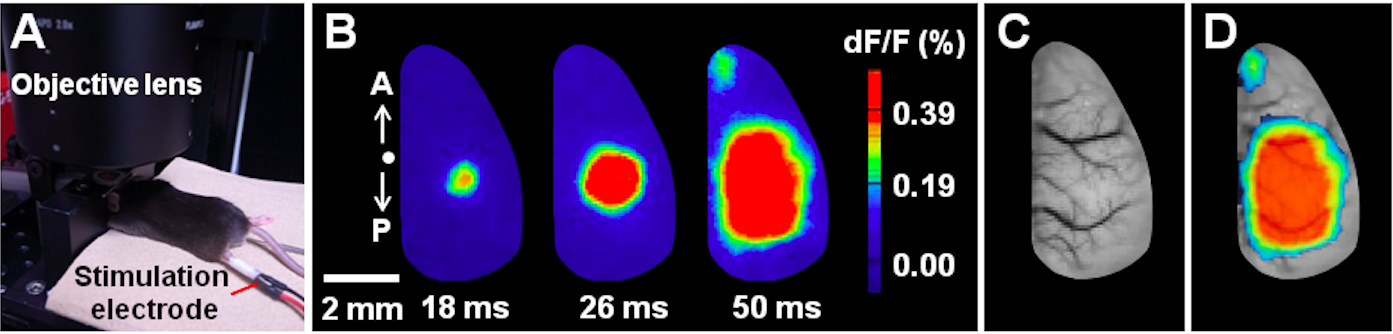
Figure 5. Example of VSD imaging under anesthesia. A. Experimental configuration during the VSD imaging under anesthesia. B. Spatiotemporal dynamics of cortical VSD responses evoked by contralateral hindpaw stimulation (single pulse, 0.1 ms duration, 100 V) under anesthesia. The time post-stimulation is indicated. Images were collected with 2-ms temporal and 80-μm spatial resolution. The letters A and P denote anterior and posterior directions, respectively. A dot indicates the bregma location. C. The superficial blood vessels in the imaging area. D. Overlaid image.
- Record cortical activity (Figure 5A). Electrically stimulate the contralateral hindpaw 3 sec after starting the imaging, and monitor the VSD responses for 4 sec (3 sec before stimulation and 1 sec after stimulation) at every imaging (Figure 5B). By referencing the positions of blood vessels captured beforehand (step C13; Figure 5C), overlay the cortical activity map and vessel positions in order to identify a functional cortical map (Figure 5D).
Notes
To prevent phototoxicity of the dye to neurons, the intensity of the excitation light and exposure time should be minimized. We could obtain stable imaging for 2 h when we use 4 sec exposure time every 40 sec. Proper washing of the unbinding dye is helpful in improving the single-to-noise ratio. However, excessive washing decreases stable imaging time.
Recipes
- Ringer’s solution (pH 7.2 with NaOH)
Dissolve 7.89 g NaCl (135 mM), 0.40 g KCl (5.4 mM), 0.20 g MgCl2.6H2O (1 mM), 0.26 g CaCl2.2H2O (1.8 mM), and 1.19 g HEPES (5 mM) in distilled water, for a total volume of 1 L
Stored at 4 °C
Warm to body temperature before use - Voltage-sensitive dye (VSD) solution (2 mg/ml)
Dissolve 10 mg RH1691 in the 5-ml ringer’s solution, and mix with a vortex mixer
Stored at -20 °C, and keep in the dark
Warm to body temperature, and mix with a vortex mixer before use
Acknowledgments
This protocol was adapted from Manita et al. (2015). The authors acknowledge support from a Grant-in-Aid for Young Scientists (A) from the JSPS (Japan Society for the Promotion of Science), the Uehara Memorial Foundation, and the Japan Prize Foundation to M. M.; from a Grant-in-Aid for Challenging Exploratory Research from the JSPS to T. S.
References
- Manita, S., Suzuki, T., Homma, C., Matsumoto, T., Odagawa, M., Yamada, K., Ota, K., Matsubara, C., Inutsuka, A., Sato, M., Ohkura, M., Yamanaka, A., Yanagawa, Y., Nakai, J., Hayashi, Y., Larkum, M. E. and Murayama, M. (2015). A top-down cortical circuit for accurate sensory perception. Neuron 86(5): 1304-1316.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Suzuki, T. and Murayama, M. (2016). Craniotomy for Cortical Voltage-sensitive Dye Imaging in Mice. Bio-protocol 6(3): e1722. DOI: 10.21769/BioProtoc.1722.
Category
Neuroscience > Neuroanatomy and circuitry > Live-cell imaging
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link