- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation of FAP Cells from Mouse Dystrophic Skeletal Muscle Using Fluorescence Activated Cell Sorting
Published: Vol 4, Iss 22, Nov 20, 2014 DOI: 10.21769/BioProtoc.1292 Views: 15370
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Selective Enrichment and Identification of Cerebrospinal Fluid-Contacting Neurons In Vitro via PKD2L1 Promoter-Driven Lentiviral System
Wei Tan [...] Qing Li
Nov 20, 2025 1335 Views

A Simplified 3D-Plasma Culture Method for Generating Minimally Manipulated Autologous Equine Muscle-Derived Progenitor Cells
Hélène Graide [...] Didier Serteyn
Dec 5, 2025 1251 Views

Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
Jianwei Li [...] Guohui Lu
Dec 5, 2025 1460 Views
Abstract
A population of muscle resident CD45-, CD31- cells expressing the mesenchymal PDGF receptor alpha (PDGFRα) as well as Sca-1 was first isolated in healthy mouse muscles in Uezumi et al. (2010). In the same year, Joe et al. (2010) identified and purified fibro-adipogenic precursors (FAPs), cells located into the interstitial space between myofibers close to vessels, negative for CD45, CD31,α7-Integrin, but expressing CD34, Sca-1.
Both groups demonstrated that these cells are not myogenic in vitro or in vivo, but they are capable of differentiating in vitro towards both fibrogenic and adipogenic lineage (Uezumi et al., 2011). Further marker analysis indicates that the two groups identified independently the same cell population (Natarajan et al., 2010).
FAPs are an important source of fibrosis and adipogenesis in dystrophic skeletal muscle (Natarajan et al., 2010; Cordani et al., 2014). We have recently demonstrated that Nitric Oxide regulates FAP fate inhibiting in vitro their differentiation into adipocytes. In mdx mice, an animal model of DMD, fed with a diet containing the nitric oxide donating drug, Molsidomine, the number of PDGFRα+ cells was reduced as well as the deposition of both skeletal muscle fat and connective tissues (Cordani et al., 2014). Here we described a method to isolate in both wild type and in mdx dystrophic muscle pure population of FAPs by double selection for SCA-1 and PDGFRα positivity in absence of the satellite cell markers SM/C2.6 and α7integrin as well of the pan-lymphocytes marker CD45 or endothelial marker CD31.
Materials and Reagents
- 8-10 weeks old mice C57BL/6J wild-type mice (Charles River Laboratories International, http://www.criver.com) and mdx-4cv mice (B6Ros.Cg-Dmdmdx-4cv/J, crossed on C57/BL/6 background; Jackson ImmunoResearch Laboratories)
Note: Animals were treated in accordance with European Community guidelines and with the approval of the Institutional Ethical Committee. - Collagenase II (Worthington Biochemical, catalog number: CLSS2 )
- Dulbecco's modified high glucose Eagle's medium (DMEM high glucose) (EuroClone, catalog number: ECB7501L )
- 100 U/ml penicillin and 100 μg/ml streptomycin (EuroClone)
- L-glutammine (EuroClone)
- Recombinant human basic fibroblast growth factor (b-FGF) (Pepro Tech, catalog number: 100-18B )
- Growth factor reduced BD MatrigelTM Matrix (BD Biosciences, catalog number: 356230 )
- Foetal Bovine Serum (FBS) (EuroClone)
- Sterile phosphate buffered saline (PBS) w/o Ca++Mg++ (EuroClone)
- Antibodies
- Anti-CD31-phycoerythrin/Cy7 (anti-CD31-PE/Cy7, clone 390) (eBioscience, catalog number: 25-0311 )
- Anti-CD45-PECy7 (clone 30-F11) (eBioscience, catalog number: 15-0451 )
- Anti-SM/C2.6-Biotin (kindly provided by Dr. Fukada) (Fukada et al., 2004)
- Streptavidin-PE (BioLegend)
- Anti-α7-Integrin-PE (clone R2F2) (AbLab Laboratorio di Istologia e Citologia Patologica Veterinaria, catalog number: AB10RS24MW215 )
- Anti-PDGFRα-allophycocyanin (APC, CD140a, clone APA5) (BioLegend, catalog number: 135907 )
- Anti-LY-6A/E SCA-1-allophycocyanin/Cy7 (APC/Cy7, clone B7) (BD Biosciences, catalog number: 560654 )
- 7-aminoactinomycin D (7-AAD) (Life Technologies, catalog number: A1310 )
- Anti-CD31-phycoerythrin/Cy7 (anti-CD31-PE/Cy7, clone 390) (eBioscience, catalog number: 25-0311 )
- Growth medium (GM) (see Recipes)
- Wash buffer (WB) (see Recipes)
- Collagenase II solution (see Recipes)
- Erythrocytes lysis buffer (see Recipes)
Note: Use 1 ml of this solution for approximately 1 g of muscle. - Sorting buffer (see Recipes)
- Matrigel solution (see Recipes)
- b-FGF solution (see Recipes)
Equipment
- Scissors and tweezers
- Cell culture plastic dishes (Corning, Costar®)
- Six multiwells (Corning, Costar®)
- Centrifuge
- 50 and 15 ml plastic tubes
- 18G-10 ml syringes
- 70 μm and 40 μm cell strainer caps (BD Biosciences)
- Beckam Coulter Cell Sorter MoFloTM XDP (Beckman Coulter, catalog number: ML99030 )
- Cell culture incubator at 37 °C and 5% CO2
- Microscope or cell counter
Procedure
- Sacrifice the mice (C57BL/6 or MDX) by delivering increasing concentrations of CO2 and remove hindlimb muscles.
- Weigh the muscle mass.
- Leave them in cold PBS-containing dish.
- Remove visible tendon, adipose tissue and vessel.
- Mince the muscles on new dish (not containing PBS) using a curved tip scissor for few minutes until the tissue appears like a mush.
- Transfer the tissue into a 50 ml plastic tube and add 0.2% collagenase II in DMEM (serum-free). Volume to use is 2-4 ml of collagenase for 1 g of muscle weight: Usually each mouse allows obtaining 1-1.5 g of tissue. Put plastic tubes into a shaking thermostatic bath, at 37 °C for 1 h.
- Separate undigested from digested material by centrifugation at 500 rpm. All the centrifugations are at room temperature. Supernatant (digested material) was removed, diluted with room temperature PBS (approximately 50 ml of PBS/1 g muscle) and go through a 18 G-10 ml syringe to create a single cell suspension (about 10 passages through the needle).
- Add to the un-digesting muscle fresh collagenase solution (using the same volume of step 6) and leave in the shaking thermostatic bath, at 37 °C for additional 30 min. Then, repeat step 7. Discard eventually indigested material that may remain after the second centrifugation procedure.
- Muscle slurries obtained by the two digestions were pulled and filtered through 70 μm cell strainer cap and subsequently through 40 μm cell strainer cap, using 50 ml syringes and 50 ml plastic tubes.
- Centrifuge at 2,000 rpm for 10 min and remove supernatant using a pipette.
- Eliminate blood red cells by re-suspending cell with 1-2 ml of erythrocyte lysis buffer.
- Dilute erythrocyte lysis buffer by addiction of 50 ml of PBS containing 2% FBS and centrifuge 2,000 rpm for 10 min at room temperature.
- After removing the supernatant, using a pipette, add 1 ml PBS containing 10% of FBS and count the cells.
- Centrifuge tubes 2,000 rpm for 10 min and incubate recovered cells for 30 min with anti-SM/C2.6-Biotin on ice. Use 1 µl of antibodies for 1 x 106 cells in 100 µl of PBS containing 2% of FBS.
- Add 1 ml wash buffer and centrifuge 2,000 rpm for 5 min.
- After removing the supernatant, incubate on ice for 30 min with Streptavidin-PE and with the other labeled antibodies [anti-CD31-PECy7 and anti-CD45-PECy7, anti-α7-Integrin-PE (for MDX mice), anti-PDGFRα-APC, anti-LY-6A/E SCA-1-APC/Cy7] and with 7-aminoactinomycin D. As in step 14, use 1 µl of antibodies for 1 x 106 cells in 100 µl of PBS containing 2% of FBS.
- Add 50 ml of wash buffer and centrifuge 2,000 rpm for 5 min.
- After removing the supernatant, add 2 ml of sorting buffer/mice.
- To set up the instrument, sample of about 50-100,000 cells were in parallel individually stained with the each single antibody or with 7-aminoactinomycin (single staining for compensation) in the same conditions of samples to sort. A sample of 50-100,000 cells was also no incubated with any antibody (unstained control). After 30 min, add 1 ml of wash buffer then centrifuge (2,000 rpm for 5 min). Remove supernatant and add 200-300 µl of PBS containing 2% of FBS.
- For cell sorter setting: Using unstained control, exclude debris and dead cells by forward scatter and side scatter and set basal fluorescence (Figure 1A). Then make 7-AAD gating using sample stained with 7-aminoactinomycin (gate 1, 7AAD negative cells and gate 2) to further eliminate dead ells.
- Compensate fluorescence using the samples individually stained with each antibodies and then select in sample to sort FAP cells as CD45-/CD31- (gate 3)/smc2.6- or α7integrin- (gate 4)/SCA-1+ and PDGFRα+ (gate 5) cells. To improve purity, we preferred to employ anti- smc2.6 for WT mice and anti-α7integrin for mdx mice.
- At the end of sorting, plate FAP cells at 1 x 104 cells/cm2 in multiwell plates previously coated with Matrigel and culture for 6 days in growth medium plus b-FGF in a humidified incubator (37 °C and 5% CO2).
Representative data
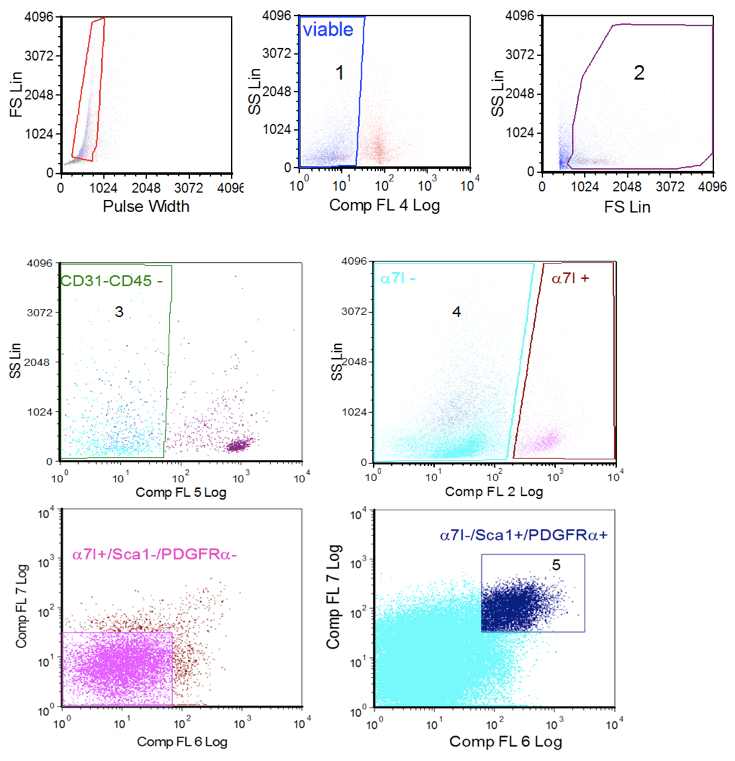
Figure 1. Cell sorter procedure. A. single cells gating; B. viable cells gating based on selection of 7ADD negative cells (1, left) and forward scatter/side scatter parametes (2, right); C. CD31 and CD45 negative cells gating (3); D. α7-integrin gating (4); E. satellite cells gating (Sca 1/PDGFRα negative cells from α7-integrin positive gate); F. FAP cells gatining (ScA 1/PDGFRα positive cells from the α7-intergrin negative gate (5).
Notes
- FAP cells recovery range from 1 to 3% of total cells. Digestion seems to be the critical point.
Recipes
- Growth medium (GM)
Dulbecco's modified high glucose Eagle's medium (DMEM) supplemented with heat inactivated 20% fetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin plus 5 ng/ml of recombinant human basic fibroblast growth factor - Wash buffer (WB)
PBS w/o Ca++ and Mg++ with heat inactivated 2% FBS - Collagenase II solution
0.2% collagenase solution was prepared in DMEM, filtered on a 0.2 µm filter and immediately used. - Erythrocytes lysis buffer
0.8% NH4Cl in Tris-buffer (pH 8) - Sorting buffer
PBS w/o Ca2+ and Mg2+ with 5% heat inactivated FBS - Matrigel solution
Dilute Matrigel 1:100 in DMEM and use immediately after preparation for plate coating
Left Matrigel working solution into the plate for 30 min before removing
Matrigel must not dry - b-FGF solution
Prepare 100 µg/ml stock solution aliquots and keep them at 20 °C
In culture b-FGF concentration was 5 ng/ml
After defrosting, b-FGF aliquot could remain at 4 °C and used within a week
Acknowledgments
We are grateful to Prof. So-ichiro Fukada (Osaka University, Osaka, Japan) for the SMC/2.6 antibody. This work was supported by the European Community’s framework program FP7/2007-2013 under grant agreement n° 241440 (ENDOSTEM), the Italian Ministry of Health RC 2013, Associazione Italiana Ricerca sul Cancro (AIRC IG11362) and from the Ministero della Università e Ricerca PRIN 2010-2011.
References
- Cordani, N., Pisa, V., Pozzi, L., Sciorati, C. and Clementi, E. (2014). Nitric oxide controls fat deposition in dystrophic skeletal muscle by regulating fibro-adipogenic precursor differentiation. Stem Cells 32(4): 874-885.
- Fukada, S., Higuchi, S., Segawa, M., Koda, K., Yamamoto, Y., Tsujikawa, K., Kohama, Y., Uezumi, A., Imamura, M., Miyagoe-Suzuki, Y., Takeda, S. and Yamamoto, H. (2004). Purification and cell-surface marker characterization of quiescent satellite cells from murine skeletal muscle by a novel monoclonal antibody. Exp Cell Res 296(2): 245-255.
- Joe, A. W., Yi, L., Natarajan, A., Le Grand, F., So, L., Wang, J., Rudnicki, M. A. and Rossi, F. M. (2010). Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 12(2): 153-163.
- Natarajan, A., Lemos, D. R. and Rossi, F. M. (2010). Fibro/adipogenic progenitors: a double-edged sword in skeletal muscle regeneration. Cell Cycle 9(11): 2045-2046.
- Uezumi, A., Fukada, S., Yamamoto, N., Takeda, S. and Tsuchida, K. (2010). Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol 12(2): 143-152.
- Uezumi, A., Ito, T., Morikawa, D., Shimizu, N., Yoneda, T., Segawa, M., Yamaguchi, M., Ogawa, R., Matev, M. M., Miyagoe-Suzuki, Y., Takeda, S., Tsujikawa, K., Tsuchida, K., Yamamoto, H. and Fukada, S. (2011). Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci 124(Pt 21): 3654-3664.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Cordani, N., Pisa, V., Pozzi, L. and Sciorati, C. (2014). Isolation of FAP Cells from Mouse Dystrophic Skeletal Muscle Using Fluorescence Activated Cell Sorting. Bio-protocol 4(22): e1292. DOI: 10.21769/BioProtoc.1292.
Category
Stem Cell > Adult stem cell > Muscle stem cell
Cell Biology > Cell isolation and culture > Cell isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










