- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Analysis of Total Se Content in Rice
Published: Vol 4, Iss 19, Oct 5, 2014 DOI: 10.21769/BioProtoc.1248 Views: 8460
Reviewed by: Tie LiuAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Targeting Ultrastructural Events at the Graft Interface of Arabidopsis thaliana by A Correlative Light Electron Microscopy Approach
Clément Chambaud [...] Lysiane Brocard
Jan 20, 2023 2973 Views

Isolation of Intact Vacuoles from Arabidopsis Root Protoplasts and Elemental Analysis
Chuanfeng Ju [...] Zhenqian Zhang
Mar 5, 2023 2035 Views

High-Performance Liquid Chromatography Quantification of Glyphosate, Aminomethylphosphonic Acid, and Ascorbate in Culture Medium and Microalgal Cells
Juan Manuel Ostera [...] Gabriela Malanga
Apr 5, 2025 1171 Views
Abstract
Total Se content in rice is normally low and it is difficult to determine it exactly because of Se volatilization and pollution during the digestion process. In this method, rice sample is digested thoroughly and Se volatilization is reduced greatly by designing a specific digestion tube, increasing digestion temperature by three steps, controlling the amount of mixed acid and adjusting the location of digestion tube in the digestion furnace. Se pollution is also reduced greatly by specific cleaning treatments.
Materials and Reagents
- Rice sample: Polished rice or brown rice
- 10% HCl (Sigma-Aldrich, catalog number: 7647-01-0 )
- 65% HNO3 (Sigma-Aldrich, catalog number: 7697-37-2 )
- 72% HClO4 (Sigma-Aldrich, catalog number: 7601-90-3 )
- 6 mM HCl (Sigma-Aldrich, catalog number: 7647-01-0) (used for the reduction of SeO42- to SeO32-)
- Selenium standard solution (Sigma-Aldrich, catalog number: 7782-49-2 ) (1, 5, 10, 20, 50, and 100 μg/L)
- Acid mixture: HNO3: HClO4 (V/V) = 4:1
Equipment
- Digestion tube (see Design digestion tube)
- 40 mesh nylon sieve (15 cm diameter)
- A paper bag (8 x 10 cm)
- Parafilm (Parafilm, catalog number: PM-996 )
- Volumetric flask (25 ml)
- Glass stopper (match 25 ml volumetric flask)
- Atomic fluorescence spectrometer (AFS) (model number: BRAIC AFS 610A )
- Inductively coupled plasma mass spectrometry (ICP-MS) (PerkinElmer, model number: ELAN-DRCe )
Procedure
- Design digestion tube
- The digestion tube is designed 300 mm in length. The edge of tube mouth is thickened.
- The outer- and inner-diameter of tube mouth is 28 mm and 21 mm, respectively.
- The thickness of digestion tube mouth is 3.5 mm. The outer diameter of digestion tube is 26 mm, the thickness is 2 mm.
- The digestion tube neck is at 25 mm from tube mouth. The outer diameter of tube neck is 18.5 mm, the length is 15 mm. The thickness of digestion tube at the bottom is 3.5 mm.
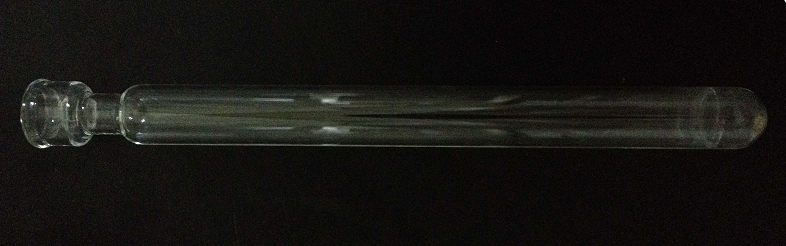
Figure 1. The structure of digestion tube
- The digestion tube is designed 300 mm in length. The edge of tube mouth is thickened.
- Clean digestion tube
- Digestion tubes are initially soaked in running water added a small amount of cleaning solvent.
- Put the tube brush into the bottom of digestion tube, scrub rotatablely the inwall up and down. Funnel is rotatablely scrubed with tube brush. Add diluted cleaning solvent into volumetric flask, then clean ultrasonically for 30 min.
- After cleaning, the digestion tubes, funnels and volumetric flasks are rinsed with deionized water (>18 MΩ) for 2-3 times, then put into 10% HCl and soak for more than 24 h, respectively.
- After being taken out, digestion tubes and the funnels are scrubed with brush again and rinsed with deionized water for 2-3 times, respectively. Volumetric flasks are directly rinsed with deionized water.
- Then, digestion tubes and volumetric flasks are inverted on special shelf until no drop of water appear inwall. At last, digestion tubes and volumetric flasks are oven-dried at 60 °C.
- Digestion tubes are initially soaked in running water added a small amount of cleaning solvent.
- Digestion process
- Take about 15-20 g of rice sample, and grind to a powder in a mortar, then pass through 40 mesh nylon sieve and put in a paper bag. After grinding one sample, please open the mortar and take out the nylon sieve, then clean them throughly with a brush.
- About 0.5 g of rice powder sample is weighed, and make the digestion tube lean and add it into the bottom. Then 5 ml of an acid mixture of 4 ml HNO3 and 1 ml HClO4 is added slowly along the tube wall, is shaken gently to mix sample and mixed acid throughly.
- Seal the digestion tube mouth with single layer parafilm. Put it in a fume hood for over 24 h. Fume hood wall and the top are washed with a wet cloth before digestion. All the reagents used are high-pure grade.
- Place the digestion tubes into the holes of digestion furnace. All the samples are initially digested for 30 min at 60 °C and continue to digest for 60 min at 100 °C.
- Then, add 2.5 ml of mixed acid into digestion tubes and raise the temperature to 150 °C. Similarly, at least 3 of blank samples and standard tea material samples (GSV-4, 0.072 mg Se/kg, GBW07605) are simultaneously digested with the test samples for quality control, respectively.
- The locations of digestion tubes in the digestion furnace are continually adjusted during the digestion process to keep the digested liquid surface of each tube at the same height.
- The digestion process is over until the brown acid fog disappears and the white fog appears in the digestion tubes. Then the digestion tubes are cooled to 100 °C, add 2.5 ml of 6 mM HCl to reduce SeO42- to SeO32-, and continue to heat at 100 °C until brown acid fog disappears. If Se content is determinated by inductively coupled plasma mass spectrometry (ICP-MS), the reduction of Se by hydrochloric acid is not needed. It will take about 6-8 h to finish the whole digestion process according to the amount of sample.

Figure 2. The digestion state of standard tea material samples
- Take about 15-20 g of rice sample, and grind to a powder in a mortar, then pass through 40 mesh nylon sieve and put in a paper bag. After grinding one sample, please open the mortar and take out the nylon sieve, then clean them throughly with a brush.
- Measure Se content
- After the temperature reduce to room temperature, the digestion tubes are gently removed from the holes of digestion furnace and placed in the digestion tube shelf. The digests are transferred into the volumetric flasks, the digestion tubes are rinsed with deionized water for 3-4 times. The rinsing solution is also added into the volumetric flask until a final volume reaches to 25 ml, then cover it closely with glass stopper.
- Place the bottom of the volumetric flask on a vortex and rotate for 30 sec and mix throughly. Put aside for over 2 h before Se determination.
- Selenium standard solution (10 mg/L) is gradually diluted to concentrations of 1, 5, 10, 20, 50, and 100 μg/L with 1% HNO3, respectively.
- Then Se content is determined by atomic fluorescence spectrometer (AFS) or by ICP-MS. The standard curve of Se content is drawn. Se content of the test samples are also determined.
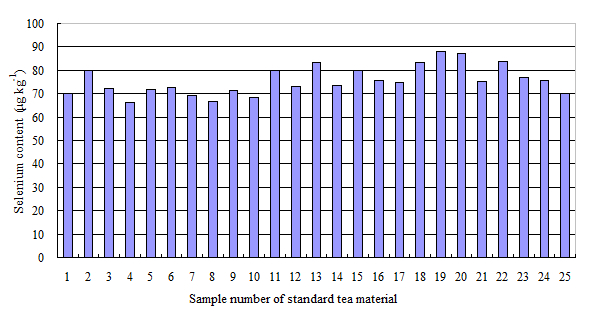
Figure 3. Se content of standard tea material samples
- The parameters for the determination of Se by AFS is as follows: Wavelength 196 nm, PMT voltage 280 v, HCl main cathode current 80 mA, Carrier gas flux 300 ml/min, Sampling volume 1.0 ml, Atomizer heigth 7 mm, Sampling pump rate 100 r/min, Sampling time 18 sec; The parameters for determination of Se by ICP-MS is as follows: Power 1,300 W, Plasma gas flow rate 15 L/min, Auxiliary gas flow rate 0.2 L/min, Atomizer flow rate 0.89 ml/min. Reaction gas methane flow rate 0.6 ml/min.
- After the temperature reduce to room temperature, the digestion tubes are gently removed from the holes of digestion furnace and placed in the digestion tube shelf. The digests are transferred into the volumetric flasks, the digestion tubes are rinsed with deionized water for 3-4 times. The rinsing solution is also added into the volumetric flask until a final volume reaches to 25 ml, then cover it closely with glass stopper.
Notes
- The edge of tube mouth and the bottom is thickened to prevent to be broken.
- The digestion tube neck is made thin at 25 mm from the tube mouth to increase the condensation of acid fog and reduce the volatilization of mixed acid.
- The amount of rice sample is controlled within about 0.5 g to reduce the digestion time.
- The total amount of mixed acid added into digestion tube is controlled below 7.5 ml. The digestion temperature is controlled at 150 °C. Thus, the digest solution is avoided to splash on the tube wall.
- The location of digestion tube in the digestion furnace is needed to adjust to keep the digest solution at the same height in each tube.
Acknowledgments
This work has been published in Zhang et al. (2014). The work was supported by the 43rd China Postdoctoral Science Foundation (No: 20080430588), the talent foundation (No: 09001107) and research foundation from Henan University of Science and Technology (No: 13560036). We thank Hongzhi Zhang (Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences) for selenium content analysis using ICP-MS.
References
- Zhang, L.*, Hu, B.*, Li, W., Che, R., Deng, K., Li, H., Yu, F., Ling, H., Li, Y. and Chu, C. (2014). OsPT2, a phosphate transporter, is involved in the active uptake of selenite in rice. New Phytol 201(4): 1183-1191. (*Co-first authors)
- Zhang, L., Shi, W. and Wang, X. (2006). Difference in selenite absorption between high-and low-selenium rice cultivars and its mechanism. Plant and Soil 282(1-2): 183-193.
- Zhang, L., Shi, W., Wang, X. and Zhou, X. (2006). Genotypic differences in selenium accumulation in rice seedlings at early growth stage and analysis of dominant factors influencing selenium content in rice seeds. J Plant Nutrition 29(9): 1601-1618.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Zhang, L., Yu, F., Deng, K., Hu, B. and Chu, C. (2014). Analysis of Total Se Content in Rice. Bio-protocol 4(19): e1248. DOI: 10.21769/BioProtoc.1248.
Category
Plant Science > Plant physiology > Ion analysis
Plant Science > Plant biochemistry > Other compound
Plant Science > Plant physiology > Tissue analysis
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











