- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Immuno-EM Analysis of PF13_0191-GFP Expressing Parasites
Published: Vol 4, Iss 11, Jun 5, 2014 DOI: 10.21769/BioProtoc.1137 Views: 9919
Reviewed by: Kanika GeraFanglian HeAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Quantification of Cutaneous Ionocytes in Small Aquatic Organisms
Garfield T. Kwan [...] Martin Tresguerres
May 5, 2019 5891 Views

Non-separate Mouse Sclerochoroid/RPE/Retina Staining and Whole Mount for the Integral Observation of Subretinal Layer
Sung-Jin Lee and Soo-Young Kim
Jan 5, 2021 4451 Views

Immunofluorescence for Detection of TOR Kinase Activity In Situ in Photosynthetic Organisms
Ana P. Lando [...] Giselle M. A. Martínez-Noël
Dec 20, 2024 1811 Views
Abstract
This protocol was used to prepare pre-embedding samples of Plasmodium falciparum blood stage parasites that overexpressed the parasite protein PF13_0191 tagged with GFP. Using GFP-specific antibodies and Protein A-Gold the localisation of the overexpressed protein in the infected host cell was determined using standard transmission electron microscopy (EM). Pre-embedding EM is a common method where the antibodies are introduced before embedding and sectioning. This method avoids the problem that antigens are often difficult to detect on EM-sections after embedding. In the method described here antigens in the parasite-infected host cell are detected. Entry of the antibody is made possible through permeabilisation of the host cell with tetanolysin. In principle this method could also be used to detect antigens within the parasite if the sample is appropriately fixed and permeabilised before addition of the relevant antibody. While access of the antibody will avoid the detection problems often seen with post-embedding methods, this procedure will produce comparably poorer morphology.
Materials and Reagents
- Parasite culture
- Plasmodium falciparum wildtype 3D7 parasites transfected with your protein of interest tagged with GFP
- Sterile, human 0+ erythrocyte concentrate (Blood Banking)
- RPMI complete medium (see Recipes)
- RPMI-1640 (AppliChem GmbH, catalog number: A1538,9010 )
- NaHCO3 (Sigma-Aldrich, catalog number: S5761 )
- Glucose (Merck KgaA, catalog number: 1.08342.1000 )
- Albumax II (Life Technologies, Gibco®, catalog number: 11021-037 )
- Hypoxanthine (Sigma-Aldrich, catalog number: H9636 )
- Gentamicin (Ratiopharm GmbH)
- RPMI-1640 (AppliChem GmbH, catalog number: A1538,9010 )
- Plasmodium falciparum wildtype 3D7 parasites transfected with your protein of interest tagged with GFP
- Pre-embedding samples
- Percoll (GE Healthcare, catalog number: 17-0891-02 )
- Formaldehyde (Polysciences)
- Tetanolysin (List Biological Labs, catalog number: 199 )
- BSA (Enzo Life Sciences, Biomol®)
- Mouse anti-GFP (Roche Diagnostics, catalog number: 11814460001 )
- Rabbit anti-mouse linker antibodies (Dako, catalog number: Z0259 )
- Protein A-Gold (6 nm) (Aurion, catalog number: 806-111 )
- 25% Glutaraldehyde (Electron Microscopy Sciences, catalog number: 16210 )
- OsO4 (Electron Microscopy Sciences, catalog number: 19152 )
- Low melting agarose (Electron Microscopy Sciences, catalog number: 10207 )
- 100% ethanol (Merck KGaA)
- Propylene oxide (Science Services GmbH, catalog number: UN1280 )
- Gelantine capsules (Plano, catalog number: G29212 )
- Uranyl acetate (Agar Scientific, catalog number: R1260 )
- Methyl nadic anhydride (MNA) (Agar Scientific, catalog number: R1083 )
- Dodecenylsuccinic anhydride (DDSA) (Agar Scientific, catalog number: R1053 )
- EPON (Agar Scientific, catalog number: R1045 )
- Benzyldimethylamine (BDMA) (Agar Scientific, catalog number: R1062b )
- Pb(NO3)2 (Merck KGaA, catalog number: 12438 )
- Na(CH3)2AsO2.3H2O (Fluca)
- Saccharose (Merck KGaA)
- 10x PBS (see Recipes)
- Tetanolysin (see Recipes)
- Sodium cacodylate buffer (see Recipes)
- EPON (see Recipes)
- Lead citrate (pH 12) (Reynolds' stain) (see Recipes)
- Percoll (GE Healthcare, catalog number: 17-0891-02 )
Equipment
- Falcon tubes (15 ml, 50 ml)
- Centrifuge
- Eppendorf tubes (1.5 ml, 2 ml)
- Sterilisation filters (0.22 µm)
- Bunsen burner
- Water bath
- Glass bottles
- Thermo block
- Rolling device
- Razor blades
- Sealable glass vials
- Tweezers
- Ultramicrotome (Leica Microsystems)
- Copper grids (Plano)
- Transmission electron microscope (FEI)
Procedure
- Preparation of pre-embedding samples
- Centrifuge 4 x 10 ml of a Plasmodium falciparum culture (5-10% trophozoites and schizonts) in 15 ml falcon tubes at 500 x g for 5 min, discard the supernatant and resuspend the pellet in 4 x 10 ml 1 x PBS. Centrifuge again.
- Purify infected red blood cells from culture using a Percoll gradient as described in Bio-protocol Immuno-EM analysis of PF13_0191-GFP Expressing Parasites (steps A2a-e) (Heiber and Spielmann, 2014).
Note: Combine the 4 pellets after the first washing step into one 1.5 ml Epppendorf tube. - Resuspend the pellet in 1 ml 1x PBS containing 2% formaldehyde and incubate at room temperature for 10 min on a rolling device.
- Centrifuge at 300 x g for 3 min and wash the pellet 3x in 1 ml 1x PBS.
- Resuspend the pellet in 800 µl 1x PBS, add 8 µl tetanolysin (1 µg/µl) and mix the solution immediately by flipping the tube with your finger. Incubate at 37 °C for 30 min and prevent the cells from sinking to the bottom by flipping the tube with your finger every few minutes.
- Centrifuge at 300 x g for 3 min and wash the pellet once by resuspending it in 1 ml 1x PBS followed by centrifuge at 300 x g for 3 min.
- Resuspend the pellet again in 1 ml 1x PBS containing 2% formaldehyde and incubate at room temperature for 5 min on a rolling device.
- Centrifuge at 300 x g for 3 min and wash the pellet once by resuspending it in 1 ml 1x PBS followed by centrifuge at 300 x g for 3 min.
- Resuspend the pellet in 500 µl 1x PBS containing 3% BSA and incubate at room temperature for 15 min on a rolling device.
- Centrifuge at 300 x g for 3 min. Resuspend the pellet in 200 µl of freshly prepared 1:20 dilution of mouse anti-GFP antibody in 3% BSA in 1x PBS and incubate at room temperature for 1.5 h on a rolling device.
- Centrifuge at 300 x g for 3 min and wash the pellet twice in 1 ml 1x PBS.
- Resuspend the pellet in 200 µl of freshly prepared 1:25 dilution of rabbit anti-mouse linker antibody in 3% BSA in 1x PBS and incubate at room temperature for 1.5 h on a rolling device.
- Centrifuge at 300 x g for 3 min and wash the pellet twice in 1 ml 1x PBS.
- Resuspend the pellet in 200 µl of freshly prepared 1:20 dilution of protein A gold in 3% BSA in 1x PBS and incubate at room temperature for 1.5 h on a rolling device.
- Centrifuge at 300 x g for 3 min and wash the pellet twice in 1 ml 1x PBS.
- Centrifuge 4 x 10 ml of a Plasmodium falciparum culture (5-10% trophozoites and schizonts) in 15 ml falcon tubes at 500 x g for 5 min, discard the supernatant and resuspend the pellet in 4 x 10 ml 1 x PBS. Centrifuge again.
- Fixation
- Resuspend the cells in 500 µl sodium cacodylate buffer and add glutaraldehyde to 2% and incubate for 1 h at 4 °C.
Note: The sample can be stored at 4 °C until use. - Centrifuge at 6,000 x g for 3 min and wash the cells 3x with 500 µl sodium cacodylate buffer.
- Post fix the cells with 1% OsO4 in cacodylate buffer for 30 min at 4 °C.
- Centrifuge at 6,000 x g for 3 min and wash the cells 3x with 500 µl sodium cacodylate buffer.
- Prepare 3% low melting agarose by heating it over a Bunsen burner and cool down to 40 °C in a water bath.
- Resuspend the cells in the 40 °C liquid 3% low melting agarose and in a 1.5 ml Eppendorf tube spin the cells down immediately at 13,000 x g for 5 min. Let the agarose harden for 30 min at 4 °C to form a stable agarose pellet containing the infected red blood cells.
- Crop off the tip of the tube with a razor blade and remove the agarose pellet.
- Resuspend the cells in 500 µl sodium cacodylate buffer and add glutaraldehyde to 2% and incubate for 1 h at 4 °C.
- Dehydration of the sample through increasing concentrations of ethanol
- Start with 30% ethanol and incubate for 10 min. Continue by transferring the sample to glass bottles containing 50%, 70%, 80%, 90%, respectively and 3x to a new bottle with 100% ethanol and incubate each time for 10 min.
- Remove the agarose pellet from the ethanol and trim the embedded infected red blood cells with a razor blade into very small pieces (max. length of the edge ~1 mm).
- Incubate the sample 2x for 10 min in propylene oxide to fully cover the sample.
- Start with 30% ethanol and incubate for 10 min. Continue by transferring the sample to glass bottles containing 50%, 70%, 80%, 90%, respectively and 3x to a new bottle with 100% ethanol and incubate each time for 10 min.
- Embedding and sectioning
Note: All steps have to be performed in glass vials since the propylene oxide dissolves the plastic tubes and sufficient volumes need to be used to fully cover the sample.- Incubate the sample in 3:1 propylene oxide/epon resin for 15 min in a sealed glass vial.
- Incubate the sample in 1:1 propylene oxide/epon resin for 30 min in a sealed glass vial.
- Incubate the sample in 1:3 propylene oxide/epon resin overnight in a sealed glass vial.
- Replace the resin mixture with fresh pure epon resin and incubate in an unsealed glass vial for 2 h at room temperature.
- Incubate for ~15 min at 60 °C to increase the fluidity of the resin.
- Fill gelatine capsules with resin and add the sample with tweezers. Incubate the samples over night at 60 °C to harden the resin.
Note: The sample will sink to the bottom of the capsule. - Prepare 50 to 70 nm ultrathin sections with an ultramicrotome and place them on copper grids using tweezers.
- Contrast the thin sections by placing them on a drop of 2% uranyl acetate for 5 min, followed by thorough rinsing in dH2O. Then place sections on a drop of lead citrate for 5 min, rinse thoroughly in dH2O and view the samples in a transmission electron microscope at 80 kV. An example for the appearance of infected RBCs in a section prepared according to the pre-embedding method is shown in Figure 1.
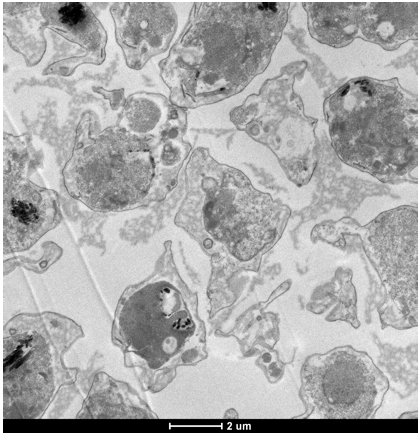
Figure 1. Example of a section of the pre-embedded parasite material. Size bar 2 µm
- Incubate the sample in 3:1 propylene oxide/epon resin for 15 min in a sealed glass vial.
Recipes
- RPMI complete medium (1 L)
15.87 g RPMI-1640
1 g NaHCO3
2 g glucose
5 g Albumax II
0.0272 g hypoxanthine
0.5 ml gentamicin (40 mg/ml)
Adjust pH to 7.2 using HCl, 1 L dH2O
Filter sterilize (0.22 µm) and stored at 4 °C - 10x PBS (1 L)
5.7 g Na2HPO4
1.25 g NaH2PO4
15.2 g NaCl
Adjust pH to pH 7.4, 1 L dH2O - Tetanolysin
Dissolved at 1 µg/µl in dH2O
Make aliquots and store at -20 °C - Sodium cacodylate buffer (0.5 L, 0.1 M, pH 7.2)
10.7 g Na(CH3)2AsO2.3H2O
17.12 g saccharose
0.111 g CaCl2
460 ml dH2O
40 ml 0.1 M HCl - EPON
13.4 g MNA
10.78 g DDSA
25.82 g EPON
Agitate for 30 min
Add 1.5% BDMA
Agitate for 5 min
Stored at -20 °C - Lead citrate (pH 12) (Reynolds' stain)
1.33 g Pb(NO3)2
1.76 g tri-Natriumcitrate.2H2O
17.12 g saccharose
30 ml H2O
Agitate for 1 min and incubate for 30 min
Add 8 ml NaOH (Solution has to be clear.)
Add H2O to 50 ml and stored in a sealed glass container (The solution is stable for 6 months.)
For use dilute 1:10 in 0.1 M NaOH
Acknowledgments
We gratefully acknowledge the work from the Tilley lab that developed the method for pre-embedding EM in selectively permeabilsed P. falciparum-infected cells that forms the basis for the procedures described here: Jackson et al. (2007). The Percoll gradient is a modified version of the protocol from Aley et al. (1986). The Percoll purification is described in more detail in the Bio-protocol 'Preparation of parasite protein extracts and Western blot analysis' (Procedure I.2.a-e).
References
- Aley, S. B., Sherwood, J. A., Marsh, K., Eidelman, O. and Howard, R. J. (1986). Identification of isolate-specific proteins on sorbitol-enriched Plasmodium falciparum infected erythrocytes from Gambian patients. Parasitology 92 ( Pt 3): 511-525.
- Heiber, A. and Spielmann, T. (2014). Preparation of parasite protein extracts and western blot analysis. Bio-protocol 4(11): e1136.
- Heiber, A., Kruse, F., Pick, C., Gruring, C., Flemming, S., Oberli, A., Schoeler, H., Retzlaff, S., Mesen-Ramirez, P., Hiss, J. A., Kadekoppala, M., Hecht, L., Holder, A. A., Gilberger, T. W. and Spielmann, T. (2013). Identification of new PNEPs indicates a substantial non-PEXEL exportome and underpins common features in Plasmodium falciparum protein export. PLoS Pathog 9(8): e1003546.
- Jackson, K. E., Spielmann, T., Hanssen, E., Adisa, A., Separovic, F., Dixon, M. W., Trenholme, K. R., Hawthorne, P. L., Gardiner, D. L., Gilberger, T. and Tilley, L. (2007). Selective permeabilization of the host cell membrane of Plasmodium falciparum-infected red blood cells with streptolysin O and equinatoxin II. Biochem J 403(1): 167-175.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Heiber, A., Retzlaff, S. and Spielmann, T. (2014). Immuno-EM Analysis of PF13_0191-GFP Expressing Parasites. Bio-protocol 4(11): e1137. DOI: 10.21769/BioProtoc.1137.
- Heiber, A., Kruse, F., Pick, C., Gruring, C., Flemming, S., Oberli, A., Schoeler, H., Retzlaff, S., Mesen-Ramirez, P., Hiss, J. A., Kadekoppala, M., Hecht, L., Holder, A. A., Gilberger, T. W. and Spielmann, T. (2013). Identification of new PNEPs indicates a substantial non-PEXEL exportome and underpins common features in Plasmodium falciparum protein export. PLoS Pathog 9(8): e1003546.
Category
Microbiology > Microbial cell biology > Cell imaging
Biochemistry > Protein > Immunodetection > Immunostaining
Cell Biology > Cell imaging > Electron microscopy
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









