Advanced Search
A Blood-retina Barrier Permeability Assay in Young Mice Using Sulfo-NHS-LC-biotin Perfusion
Published: Oct 20, 2018 DOI: 10.21769/BioProtoc.3064 Views: 4212
Abstract
Brain and retinal vasculatures exhibit restricted vascular permeability known as blood-brain barrier and blood-retina barrier. Vascular permeability can be evaluated by perfusion of the amine reactive ester derivatives of biotin such as sulfo-NHS-LC-biotin. This protocol describes experimental procedures of sulfo-NHS-LC-biotin perfusion to evaluate retinal vascular permeability. Perfused sulfo-NHS-LC-biotin remained within vessels in wild-type postnatal day 15 (P15) retinas, confirming an intact blood-retina barrier. In contrast, sulfo-NHS-LC-biotin was occasionally detected in extravascular spaces in perfused Eogt−/− retinas suggesting a partly impaired vascular integrity in the absence of Eogt (Sawaguchi et al., 2017).
Keywords: MouseMaterials and Reagents
- Microtube (Ina-optika, catalog number: ST-0150F )
- 10 cm culture dish (Corning, catalog number: 3295 )
- 6-well cell culture plate (Greiner Bio One International, catalog number: 657160 )
- Sulfo-NHS-LC-biotin (Thermo Fisher Scientific, catalog number: 21335 )
- Methanol (Wako Pure Chemical Industries, catalog number: 139-01827 )
- CF488A-conjugated streptavidin (10 mg/ml) (Biotium, catalog number: 29034 )
- Dylight 594-conjugated isolectin B4 (IB4) (Vector Laboratories, catalog number: DL-1207 )
- Vectashield® antifade mounting medium (Vector Laboratories, catalog number: H1000 )
- NaCl (Wako Pure Chemical Industries, catalog number: 191-01665 )
- KCl (Wako Pure Chemical Industries, catalog number: 163-03545 )
- Na2HPO4 (Wako Pure Chemical Industries, catalog number: 196-02835 )
- KH2PO4 (Sigma-Aldrich, catalog number: 24-5260-5 )
- Triton X-100 (Sigma-Aldrich, catalog number: T8787 )
- CaCl2 (Wako Pure Chemical Industries, catalog number: 039-00475 )
- MgCl2 (Wako Pure Chemical Industries, catalog number: 136-03995 )
- Bovine serum albumin (BSA) (MEDICAL & BIOLOGICAL LABORATORIES, catalog number: BAC61-0500 )
- Normal goat serum (NGS) (Wako Pure Chemical Industries, catalog number: 143-06561 )
- 10% formalin (Wako Pure Chemical Industries, catalog number: 060-01667 )
- 4% paraformaldehyde (PFA) (Wako Pure Chemical Industries, catalog number: 161-20141 )
- Sulfo-NHS-LC-biotin solution (see Recipes)
- 10x Calcium-magnesium free phosphate-buffered saline (CMF-PBS) (see Recipes)
- 10x Phosphate-buffered saline (PBS) (see Recipes)
- 2x Phosphate-buffered saline (PBS) (see Recipes)
- 1x Phosphate-buffered saline (PBS) (see Recipes)
- Perm/Block solution including 5% goat serum (see Recipes)
Equipment
- Forceps (Fine Science Tools, Dumont, model: #5, catalog number: 11254-20 )
- Iris scissors (Fine Science Tools, Muromachi, catalog number: 15003-08 )
- Terumo Syringe (Tuberculin), 1 ml (Terumo Medical, catalog number: 51906 )
- Terumo Syringe (Tuberculin), 10 ml (Terumo Medical, catalog number: 51904 )
- 26 G x 1/2", Regular Wall Needle (Terumo Medical, catalog number: NN-2613R )
- SZX7 Zoom Stereo Microscope (Olympus, model: SZX7 )
- Confocal microscope A1R-TiE (Nikon)
- Orbital mixer (Tokyo Rikakikai, EYELA, model: CM-1000 )
Procedure
Note: All experimental procedures were conducted in accordance with the Guidelines for Animal Experimentation in Nagoya University Graduate School of Medicine and Japanese Government Animal Protection and Management Law.
- Anesthetize P15 mice deeply by inhalation of diethyl ether and then place it in a 10 cm dish.
- Expose the mouse heart using iris scissors and forceps (Figure 1A).
- After making an incision to the right atrium using iris scissors, inject 10 ml of sulfo-NHS-LC-biotin solution into the left ventricle for 10 min at the flow rate of 1 ml/min using a 26 gauge needle connected to a 10 ml syringe (Figure 1B).
Note: The sulfo-NHS-LC-biotin dose is 0.75 μg/g body weight. P15 average weight is 6 g. - Immediately after that, inject 10 ml of 10% formalin in sodium phosphate, pH 7.4 into the left ventricle.
- Remove Eyes and transfer into a 1.5 ml tube filled with 1 ml of 4% PFA in PBS on ice (Figure 1C).
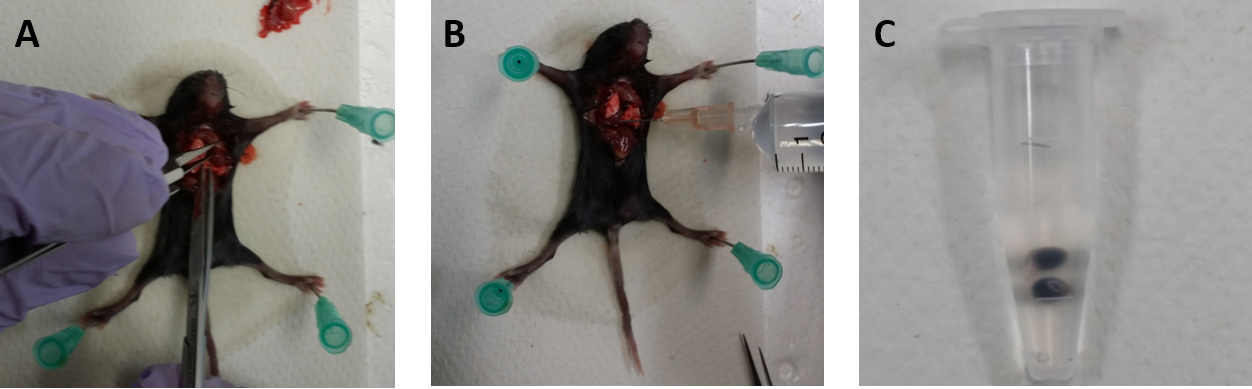
Figure 1. The procedures of mouse dissection. A. Making an incision to the right atrium using iris scissors. B. Sulfo-NHS-LC-biotin followed by PBS-CMF are injected to the left ventricle. C. The eyes are removed and transferred into a 1.5 ml microtube filled in 4% PFA in PBS. - After 15 min, remove the 4% PFA.
- Wash the eyes with 1x PBS.
- Subsequently, soak the eyes in 1 ml of 2x PBS for 15 min on ice.
Note: Use of 2x PBS decreases the osmotic pressure, and thus facilitate dissection of eyes. - Dissect retinas from eyes (Figures 2A and 2B) in 2x PBS under the microscope and flatten by dropping 1 ml of cold methanol (Figure 2C).
Pausing point: The retina can be stored at 4 °C in cold methanol for up to 1 week or -20 °C for 1 year.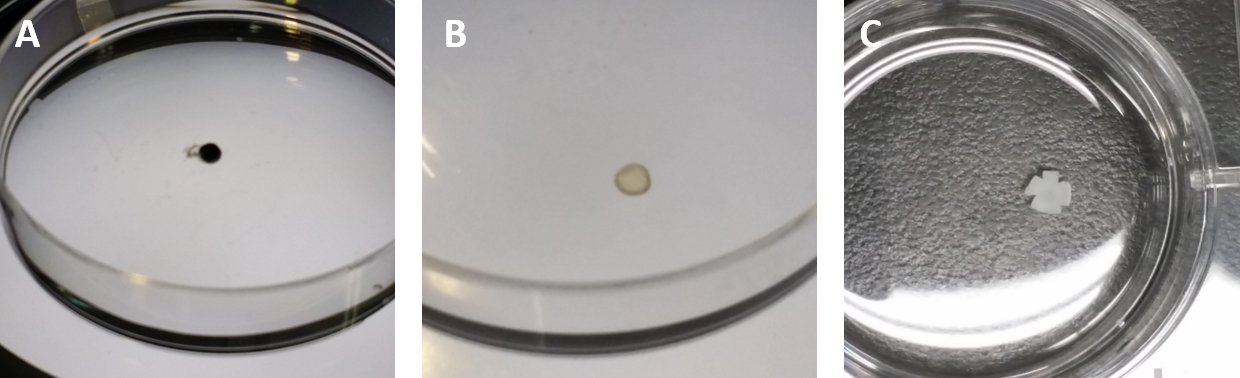
Figure 2. The procedures of obtaining and flattening a retina. A. The eye is placed in a 10 cm dish with PBS, and nerve fibers and other tissues are ready to be removed from the eyeball. B. The choroid and other tissue are removed, and only the retina is isolated. C. The retina is transferred to a 6-well plate with cold methanol. - Wash the flat retinas with PBS.
- Incubate the flat retinas in the 100 μl of Perm/Block solution for 30 min at RT or overnight at 4 °C.
- Stain the retinas with 100 μl of Perm/Block containing CF488A-conjugated streptavidin (10 g/ml) and Dylight 594-conjugated IB4 (2.5 μg/ml) for 2 h at RT or overnight at 4 °C with mixing.
Note: IB4 is a marker for endothelial cells. - After four washes with 1x PBS, mount retinas in 50 μl of Vectashield antifade mounting medium and observe by using confocal microscope A1R-TiE.
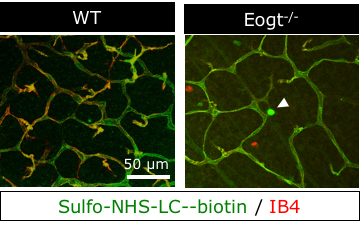
Figure 3. The confocal microscope images of P15 retinas from WT and Eogt-/- mice. Perfused sulfo-NHS-LC-biotin (green) remained within vessels (red) in wild-type P15 retinas, confirming an intact blood-retina barrier. In contrast, sulfo-NHS-LC-biotin was occasionally detected in extravascular spaces in perfused Eogt−/− retinas (arrowhead) suggesting a partly impaired vascular integrity in the absence of Eogt.
Recipes
- Sulfo-NHS-LC-biotin solution
0.75 μg/g (mouse weight) of Sulfo-NHS-LC-biotin (Thermo Fisher Scientific) dissolved in 10 ml PBS-CMF - 10x CMS-PBS
80 g NaCl
2 g KCl
14.4 g Na2HPO4
2.4 g KH2PO4
Dissolve the reagents listed above in 800 ml of distilled water. Adjust the pH to 7.4 with HCl, and then add distilled water to
1 L - 10x Phosphate-buffered saline (PBS)
80 g NaCl
2 g KCl
14.4 g Na2HPO4
2.4 g KH2PO4
1.33 g CaCl2
1.0 g MgCl2
Dissolve the reagents listed above in 800 ml of distilled water. Adjust the pH to 7.4 with HCl, and then add distilled water to
1 L - 2x Phosphate-buffered saline (PBS)
Take 10x PBS 200 ml and add distilled water to 1 L - 1x Phosphate-buffered saline (PBS)
Take 2x PBS 100 ml and add distilled water to 200 ml - Perm/Block solution including 5% goat serum
1x PBS 10 ml
5% normal serum 0.5 ml
0.3% Triton X-100 30 μl
Acknowledgments
We thank N. Toida (Nagoya Univ) for technical support. This protocol is modified from the previously published article (Chow and Gu, 2017; Sawaguchi et al., 2017). This work was supported by Japan Society for the Promotion of Science grants # JP15K15064 to TO and MO, #JP26110709 to TO, #JP26291020 to TO, #JP15K18502 to MO, #JP16J00004 to MO; Takeda Science Foundation to TO; Japan Foundation for Applied Enzymology to TO; YOKOYAMA Foundation for Clinical Pharmacology #YRY-1612 to MO. The authors declare no conflict of interest.
References
- Chow, B. W. and Gu, C. (2017). Gradual suppression of transcytosis governs functional blood-retinal barrier formation. Neuron 93(6): 1325-1333 e1323.
- Sawaguchi, S., Varshney, S., Ogawa, M., Sakaidani, Y., Yagi, H., Takeshita, K., Murohara, T., Kato, K., Sundaram, S., Stanley, P. and Okajima, T. (2017). O-GlcNAc on NOTCH1 EGF repeats regulates ligand-induced Notch signaling and vascular development in mammals. Elife 6:e24419.
Wan et al. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
Category
Cell Biology > Cell imaging > Confocal microscopy
Cell Biology > Cell staining > Cell wall
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link

